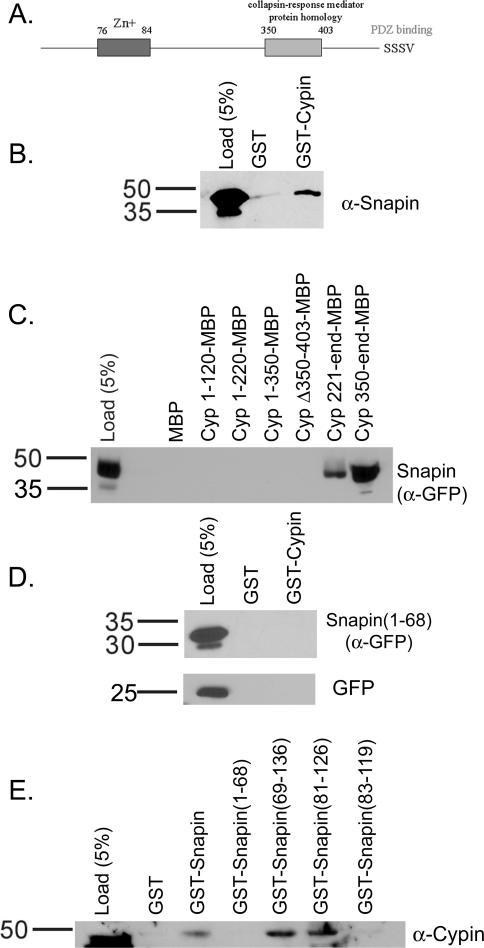
Figure 1.
The carboxyl terminal coiled-coil domain of snapin is required to bind to the CRMP homology region of cypin. (A) Schematic of cypin. (B) Detergent soluble extract of rat brain was incubated with glutathione-Sepharose bound to 25 μg of GST or GST-cypin. The Sepharose was washed and eluted, and proteins were resolved by 10% SDS-polyacrylamide electrophoresis and transferred to Immobilon-P. Western blotting of eluates demonstrates that snapin binds to cypin above background binding to GST. Similar results were found when the experiment was performed using COS-7 cells expressing snapin tagged with GFP at its carboxy terminus. (C) Amylose resin bound to 25 μg of maltose binding protein (MBP) fusions of the indicated regions of cypin were incubated with extracts from COS-7 cells expressing snapin tagged with GFP at its amino terminus. Western blotting reveals that snapin binds to cypin when the CRMP homology domain is present (221-end, 350-end) and not when it is absent (1-120, 1-220, 1-350) or deleted (Δ350-403). (D) COS-7 cells were transfected with cDNAs encoding amino acids 1–68 of snapin fused to GFP or GFP alone. Detergent-soluble extracts of these cells were incubated with glutathione-Sepharose bound to 25 μg of GST or GST-Cypin. The Sepharose was washed and eluted, and proteins were resolved by 10% SDS-polyacrylamide electrophoresis and transferred to Immobilon-P. Western blotting of eluates demonstrates that neither snapin(1-68) nor GFP binds to cypin. (E) Extracts from rat brain were incubated with GST fusions of full-length snapin, the first (1-68) and second (69-136) halves of snapin, and the carboxyl-terminal coiled-coil domain (H2) of snapin (defined as either 81-126 or 83-119). The data demonstrate that amino acids 81–126 represent the minimal binding domain of snapin that binds to cypin. Load noted represents the percentage of input material corresponding to the appropriate affinity chromatography.