Abstract
VE-cadherin is an adhesion molecule critical to vascular barrier function and angiogenesis. VE-cadherin expression levels are regulated by p120 catenin, which prevents lysosomal degradation of cadherins by unknown mechanisms. To test whether the VE-cadherin cytoplasmic domain mediates endocytosis, and to elucidate the nature of the endocytic machinery involved, the VE-cadherin tail was fused to the interleukin (IL)-2 receptor (IL-2R) extracellular domain. Internalization assays demonstrated that the VE-cadherin tail dramatically increased endocytosis of the IL-2R in a clathrin-dependent manner. Interestingly, p120 inhibited VE-cadherin endocytosis via a mechanism that required direct interactions between p120 and the VE-cadherin cytoplasmic tail. However, p120 did not inhibit transferrin internalization, demonstrating that p120 selectively regulates cadherin internalization rather than globally inhibiting clathrin-dependent endocytosis. Finally, cell surface labeling experiments in cells expressing green fluorescent protein-tagged p120 indicated that the VE-cadherin–p120 complex dissociates upon internalization. These results support a model in which the VE-cadherin tail mediates interactions with clathrin-dependent endocytic machinery, and this endocytic processing is inhibited by p120 binding to the cadherin tail. These findings suggest a novel mechanism by which a cytoplasmic binding partner for a transmembrane receptor can serve as a selective plasma membrane retention signal, thereby modulating the availability of the protein for endo-lysosomal processing.
INTRODUCTION
VE-cadherin is a member of the cadherin family of cell-cell adhesion molecules and is expressed selectively in vascular endothelial cells (Breviario et al., 1995; Dejana, 2004). Previous studies have established a key role for VE-cadherin in several processes central to endothelial physiology, including vascular barrier function and angiogenesis (Carmeliet and Collen, 2000; Stevens et al., 2000; Dejana et al., 2001; Vincent et al., 2004). Gene ablation and antibody inhibition studies have revealed a critical role for VE-cadherin in vascular patterning during development (Vittet et al., 1997; Carmeliet et al., 1999; Corada et al., 2001; Crosby et al., 2005). VE-cadherin function is also coordinated with growth factor signaling pathways that regulate endothelial proliferation during angiogenesis (Lampugnani et al., 2003; Spagnuolo et al., 2004). Furthermore, antibody blocking studies raise the possibility that cell surface VE-cadherin can be targeted by therapeutic strategies designed to limit angiogenesis associated with tumor growth (Liao et al., 2000; Corada et al., 2002; May et al., 2005). These studies demonstrate a central role for VE-cadherin in vascular biology and pathophysiology, and highlight the importance of understanding how plasma membrane levels of VE-cadherin are regulated.
Cadherins at the cell surface are associated with cytoplasmic binding partners termed catenins. The catenins bind to the carboxy-terminal tail of the cadherins and regulate cadherin cytoskeletal interactions and cadherin adhesive activity (Gooding et al., 2004). VE-cadherin is coupled to the actin cytoskeleton through interactions with β-catenin, an armadillo family protein that binds to both the cadherin tail and to actin-associating proteins such as α-catenin (Navarro et al., 1995). VE-cadherin is also coupled to the vimentin intermediate filament cytoskeleton (Kowalczyk et al., 1998; Calkins et al., 2003), and the molecules mediating these linkages seem to be critical for normal VE-cadherin adhesive activity and for VE-cadherin function during development (Valiron et al., 1996; Gallicano et al., 2001). In addition to β-catenin, the armadillo family also includes several other related molecules, including p120-catenin (Anastasiadis and Reynolds, 2000; Hatzfeld, 2005). Although not directly involved in coupling the cadherins to the cytoskeleton, p120 has emerged as a critical regulator of cadherin adhesive activity and cytoskeletal organization (Thoreson et al., 2000; Anastasiadis and Reynolds, 2001; Ozawa and Ohkubo, 2001; Pettitt et al., 2003).
In addition to regulating adhesion and actin cytoskeletal organization, recent studies revealed a core function for p120 in regulating cadherin expression levels in vertebrate cells. Separate studies demonstrated that p120 regulates steady-state levels of E-cadherin in epithelial cells (Davis et al., 2003) and VE-cadherin in vascular endothelial cells (Xiao et al., 2003a). In the absence of p120, cadherins become destabilized and targeted for degradation. The precise mechanism by which p120 regulates cadherin levels is not fully understood. However, several lines of evidence suggest that p120 regulates the entry of cell surface cadherins into a lysosomal degradation pathway (Davis et al., 2003; Xiao et al., 2003a). In the absence of p120, E-cadherin is efficiently delivered to the cell surface, but it is then rapidly turned over (Davis et al., 2003). Similarly, cell surface labeling experiments indicate that p120 regulates the lysosomal degradation of cell surface pools of VE-cadherin (Xiao et al., 2003a). These data suggest that p120 likely functions at the plasma membrane to regulate cadherin internalization. However, it is also possible that p120 regulates the fate of the cadherin after endocytosis. p120 was found to associate with kinesin and alter the rates of N-cadherin accumulation at cell-cell borders (Chen et al., 2003). Collectively, these findings strongly implicate p120 as a modulator of cadherin trafficking (Kowalczyk and Reynolds, 2004). However, these previous studies did not define a role for p120 in any specific control point in cadherin delivery to degradative compartments. Thus, it is currently unclear whether p120 regulates cadherin levels by controlling cadherin endocytosis, targeting of the protein to degradative compartments after internalization, and/or recycling back to the plasma membrane.
The present study is part of a systematic effort to delineate the role of the endocytic pathway in controlling cell surface presentation of VE-cadherin. Herein, we report that the VE-cadherin cytoplasmic domain mediates rapid and efficient endocytosis and that p120 regulates this critical step in VE-cadherin trafficking. Using a gain of function approach in which the VE-cadherin tail was fused to the interleukin 2 receptor (IL-2R) extracellular domain, we found that the cytoplasmic domain of VE-cadherin dramatically enhances IL-2R endocytosis. Furthermore, VE-cadherin internalization was found to occur through a clathrin-mediated pathway. These data indicate that the tail of VE-cadherin harbors sorting information that mediates interactions, directly or indirectly, with components of clathrin-dependent endocytic machinery. In addition, p120 blocked endocytosis mediated by the VE-cadherin tail via a mechanism that required direct interactions between p120 and the VE-cadherin juxtamembrane domain. Finally, p120 had no obvious effects on transferrin internalization, indicating that p120 does not globally inhibit clathrin-dependent endocytosis. Rather, our findings indicate that p120 functions to selectively inhibit cadherin internalization from the plasma membrane by inhibiting a process dependent upon both the cadherin cytoplasmic domain and clathrin-dependent endocytic machinery. We propose a model whereby constitutive endocytic signals intrinsic to the cadherin tail are suppressed by cytosolic p120-catenin. These findings provide new insights into the mechanisms by which p120 controls cadherin expression levels and represent the first example in which binding of a cytoplasmic protein directly inhibits endocytosis of a transmembrane receptor.
MATERIALS AND METHODS
Cell Culture
Primary cultures of dermal microvascular endothelial cells (MECs) from human neonatal foreskin were obtained from the Emory Skin Diseases Research Center (Core B) and cultured in MCDB131 medium (Invitrogen, Carlsbad, CA) as described previously (Xiao et al., 2003b). The culture medium was supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO), l-glutamine (Mediatech, Herndon, VA), cAMP (Sigma-Aldrich), hydrocortisone (Sigma-Aldrich), epidermal growth factor (Intergen, Purchase, NY), and antibiotic/antimycotic (Invitrogen). Cells were typically cultured overnight on 0.1% gelatin-coated plates and grown to 80% confluence for most experiments. For adenovirus production, human embryonic kidney cell line QBI-293A (Qbiogene, Carlsbad, CA) were routinely cultured in DMEM supplemented with 10% FBS and antibiotic/antimycotic.
Adenovirus Production
The IL-2R-VE-cadherin chimeras, p120ctn 1A, and β-catenin cDNAs were subcloned into the pAd-Track vector which coexpresses green fluorescent protein (GFP) (He et al., 1998). Adenoviruses carrying the Empty Vector (EV), VE-cadherin constructs, p120ctn 1A, β-catenin, and p0071 were produced using the pAdeasy adenovirus-packaging system as described previously (Xiao et al., 2003a; Setzer et al., 2004). For most experiments, a low dose of adenovirus was used to obtain 40–50% infection rates as monitored by GFP expression.
Immunofluorescence
MECs cultured on gelatin-coated glass coverslips were fixed in methanol for 5 min at –20°C or 3.7% paraformaldehyde for 8 min on ice followed by either extraction in 0.2% Triton X-100 for 5 min on ice or methanol for 1 min at –20°C. The choice for fixation method depends on the performance of different antibodies. Endogenous VE-cadherin was detected using mouse monoclonal antibody (mAb) directed against the VE-cadherin extracellular domain (BV-6) (Corada et al., 2001). The BV-6 antibody was fluorescently conjugated using Alexa Fluor 555 mAb labeling kit (Molecular Probes, Eugene, OR). IL-2R and the IL-2R-VE-cadherin chimeras were followed using an affinity-purified anti-IL-2R IgG produced from 7G7B6 mouse hybridoma (American Type Culture Collection, Manassas, VI) or by using an Alexa Fluor 488- or 555-conjugated anti-IL-2R IgG. VE-cadherin constructs were also followed using a chicken antibody directed against the c-myc epitope tag (Bethyl Laboratories, Montgomery, TX). The localization of p120ctn, β-catenin, p0071, and plakoglobin was determined using rabbit polyclonal antibodies against p120ctn (Santa Cruz Biotechnology, Santa Cruz, CA), β-catenin (NeoMarkers, Fremont, CA), plakoglobin (Santa Cruz Biotechnology) and p0071 (Bethyl Laboratories), respectively. The localization of early endosomes was monitored using a rabbit polyclonal early endosome antigen (EEA)-1 antibody (Affinity Bioreagents, Golden, CO). Second antibodies conjugated to various Alexa Fluors (Molecular Probes) were used for dual or triple-label immunofluorescence. Microscopy was performed using a fluorescence microscope (model DMR-E; Leica, Wetzlar, Germany) equipped with narrow band pass filters and a digital camera (model Orca; Hamamatsu, Bridgewater, NJ). Images were captured, pseudocolored, and processed using Simple PCI software (Compix, Cranberry Township, PA). In each case, the amount of vesicular VE-cadherin present was quantified by counting VE-cadherin vesicles/cell using Simple PCI software. For fluorimetry experiments, the cells were cultured on 24-well plates, and the fluorescence was measured by Synergy HT multi-detection microplate reader (KC-4; Bio-Tek Instruments, Winooski, VT).
Internalization Assay
Internalization assays were performed as described previously (Xiao et al., 2003a). MECs were cultured on glass coverslips for experimentation. Fluorescently conjugated BV-6 antibody was incubated with cells at 4°C on ice for 30 min in MCDB131 media containing 20 mM HEPES (Mediatech). Unbound antibody was removed by rinsing cells in ice-cold MCDB 131. Cells were incubated at 4°C or transferred to 37°C for various amounts of time (1–30 min) to allow the internalization of molecules from cell surface. To remove cell surface bound antibody while retaining internalized antibody, cells were acid washed for 30 min in phosphate-buffered saline (PBS), pH 2.7, containing 25 mM glycine and 3% bovine serum albumin (BSA). The cells were rinsed, fixed, and processed for immunofluorescence as described above. For experiments to monitor internalization of IL-2R or IL-2R-VE-cadherin chimeras, MECs were infected with adenovirus overnight to allow time for infection and expression of the polypeptides. The cells were surface labeled using IL-2R antibody at 4°C and transferred to 37°C for various amounts of time. In some experiments, cells were infected with EV, p120ctn, β-catenin, or p0071 4–6 h before the IL-2R-VE-cadherin chimeras to allow time for expression of the catenins. For transferrin internalization, cells were incubated with 20 μg/ml Alexa Fluor 555-conjugated transferrin (Molecular Probes) at 4°C and then allowed to internalize for 5 min. Potassium depletion was performed by hypotonic shock of the cells in hypotonic buffer (1:1 dilution of K+ depletion buffer) for 5 min at 37°C and then incubation with K+ depletion buffer (20 mM HEPES, 140 mM NaCl, 1 mM CaCl2,1 mM MgCl2, pH 7.4) for 30 min at 37°C (Wu et al., 2001). Cytosol acidification protocol was performed by incubating cells with acetic acid buffer for 15 min at 37°C (Salazar and Gonzalez, 2002).
Biotinylation Assay
MECs were grown to 80% confluence on 60-mm dishes in MEC growth media. 18 h before biotinylation, MECs were infected by adenoviruses carrying IL-2R control or the IL-2R-VE-cadcyto chimera. Cells were washed three times in ice-cold PBS+ and labeled with EZ-Link Sulfo-NHS-SS-Biotin (Pierce Chemical, Rockford, IL) at 0.5 mg/ml in PBS+, on ice, for 30 min. Excess biotin was quenched by washing twice with blocking solution (50 mM NH4Cl in PBS containing 1 mM MgCl2, and 0.1 mM CaCl2) on ice, followed by three washes in ice-cold PBS+. Cells were incubated at 4°C or transferred to 37°C for 5–30 min to allow internalization of the biotinylated surface molecules. Biotin remaining at the cell surface was stripped by 100 mM sodium 2-mercaptoethanesulfonic acid (MESNA; Sigma-Aldrich) in 50 mM Tris-HCl, pH 8.6, 100 mM NaCl, 1 mM EDTA, and 0.2% BSA as described previously (Hammond et al., 2003). The cells were then rinsed twice with ice-cold PBS+, and residual MESNA was quenched by 120 mM iodoacetamide (Sigma-Aldrich) in PBS+ (Hammond et al., 2003). After two additional rinses with ice-cold PBS+, samples were lysed using cytoskeleton (CSK) buffer (50 mM NaCl, 10 mM PIPES, 3 mM MgCl2, 300 mM sucrose, 1% Triton-X 100, 10 mM NaF, 10 mM NaP2O7, pH 6.8) containing protease inhibitor cocktail. After centrifugation at 14,000 rpm for 30 min, the supernatant was recovered and incubated with UltraLink immobilized streptavidin (Pierce Chemical) for 1 h at 4°C. Cell surface biotin-labeled proteins were captured by centrifugation, and the beads were washed three times in CSK lysis buffer. Biotin labeled proteins were released in 2× Laemmli buffer containing β-mercaptoethanol at 95°C and then processed for Western blot analysis according to standard protocols. The IL-2R extracellular domain was detected using a rabbit polyclonal antibody (Santa Cruz Biotechnology). Horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA) were used at 1:3000 dilution and detected using enhanced chemiluminescence (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The blots were quantitated using GelPlot Software in Scion Image.
RESULTS
The VE-Cadherin Cytoplasmic Tail Mediates Endocytosis
Previously, we demonstrated that VE-cadherin is constitutively internalized from the plasma membrane and delivered to the lysosome for degradation (Xiao et al., 2003b). However, the experiments lacked the temporal resolution necessary to define a function for p120-catenin in any specific step in VE-cadherin processing. To test whether the VE-cadherin cytoplasmic domain mediates endocytosis and to elucidate the nature of the endocytic machinery involved, a gain of function approach was used in which the VE-cadherin tail was fused to the IL-2R extracellular domain. The IL-2R and the IL-2R-VE-cadcyto chimera were then expressed in primary cultures of human MECs using an adenoviral delivery system. Antibodies directed against the IL-2R were used to label cell surface pools of the IL-2R or the IL-2R-VE-cadcyto chimera and the amount of internalization of the proteins was quantified. For these experiments, MECs were incubated at 4°C with the IL-2R antibody to label cell surface pools of the IL-2R or the IL-2R-VE-cadcyto chimera. The cells were then washed to remove unbound antibody and then switched to 37°C to allow for endocytosis. Cell surface-bound antibody was distinguished from internalized pools by washing cell surfaces with a low pH wash, which removes antibody bound at the plasma membrane but not antibody that has been internalized (Faundez et al., 1997).
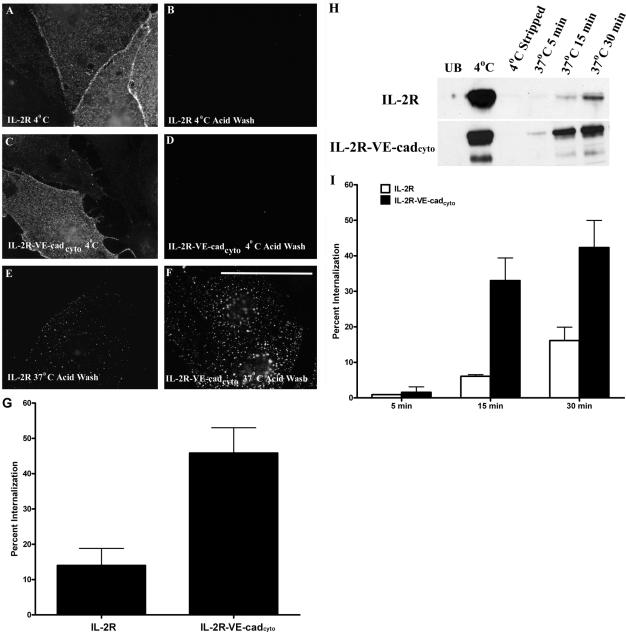
As shown in Figure 1, after labeling at 4°C with a mAb directed against the IL-2R, both the IL-2R (Figure 1A) and the IL-2R-VE-cadcyto chimera (Figure 1C) were distributed on the endothelial cell surface. In cells that remained at 4°C, acid washing removed virtually all IL-2R antibody bound to the cell surface (Figure 1, B and D). However, after incubating the cells at 37°C for 5 min, both the IL-2R (Figure 1E) and the IL-2R-VE-cadcyto chimera (Figure 1F) were detected in intracellular vesicular compartments after acid washing the cells. Interestingly, the degree of IL-2R-VE-cadcyto internalization was substantially higher (≈3-fold) than that of the IL-2R without the VE-cadherin cytoplasmic tail (Figure 1, E–G), indicating that the VE-cadherin tail accelerates endocytosis of the IL-2R. To test this possibility further, biotinylation experiments were performed to evaluate the amount of internalization of the two proteins from the cell surface. MECs expressing IL-2R or IL-2R-VE-cadcyto were biotinylated at 4°C and then transferred to 37°C for various amounts of time. Stripping buffer was then used to selectively remove biotin from cell surface proteins. Cells were then lysed and biotinylated proteins were captured using streptavidin beads. Western blot analysis using antibodies directed against the IL-2R was performed to detect the amount of IL-2R or IL-2R-VE-cadcyto internalized over time. In cells incubated at 4°C, both IL-2R-VE-cadcyto and IL-2R were expressed on the cell surface (Figure 1H). When the cells were moved to 37°C, small amounts of IL-2R internalization could be detected over time. In contrast, significant amounts of internalized IL-2R-VE-cadcyto chimera were detected as early as 15 min after incubation at 37°C. These results are consistent with the immunofluorescence based assay shown in Figure 1, A–G. This gain of function approach demonstrates that the VE-cadherin cytoplasmic tail harbors sequence information that functions to promote endocytosis.
Figure 1.
The VE-cadherin cytoplasmic tail mediates internalization. Cell surface IL-2R and IL-2R-VE-cadcyto chimera were labeled in living MECs at 4°C using a monoclonal IL-2R antibody. The cells were rinsed, fixed, and processed for immunofluorescence microscopy. A low pH wash (acid wash) was used to remove antibody bound to the cell surface but not antibody that had been internalized. In cells that remained at 4°C, acid washing the cells removed virtually all IL-2R antibody bound to the cell surface (B and D). To monitor internalization, cells were labeled at 4°C and transferred to 37°C for 5 min. After acid washing to remove cell surface antibody, cells were processed for immunofluorescence microscopy (E and F). Fluorimetry was performed to quantify the percentage of cell surface IL-2R and the IL-2R-VE-cadcyto chimera internalized after 30 min at 37°C (G). As an alternative approach, biotinylation experiments were performed (H). MECs expressing IL-2R or IL-2R-VE-cadcyto were biotinylated at 4°C and then transferred to 37°C for 5–30 min. Striping buffer was used to selectively remove biotin from cell surface proteins. Cells were then lysed and biotinylated proteins were captured using streptavidin beads. Western blot analysis using antibodies directed against the IL-2R was performed to determine the percentage of IL-2R or IL-2R-VE-cadcyto internalized over time (I). Bar, 50 μm.
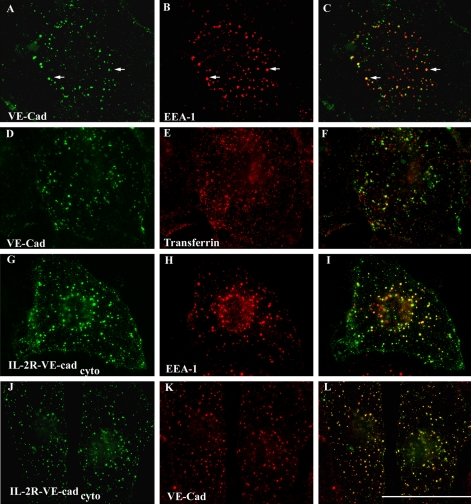
To determine whether the IL-2R-VE-cadcyto polypeptide follows the same route of internalization as endogenous VE-cadherin, colocalization experiments were carried out using surface labeled endogenous VE-cadherin and the IL-2R-VE-cadcyto chimera. As shown in Figure 2, A–C, endogenous VE-cadherin colocalized extensively with the early endosomal marker EEA-1 (Simonsen et al., 1998) after internalization. Similar to previous results with E-cadherin (Paterson et al., 2003), VE-cadherin exhibited partial colocalization with fluorescently tagged transferrin internalized from the cell surface (Figure 2, D–F). The IL-2R-VE-cadcyto chimera also exhibited extensive colocalization with EEA-1 upon internalization from the cell surface (Figure 2, G–I). Furthermore, the IL-2R-VE-cadcyto chimera exhibited striking colocalization with endogenous VE-cadherin internalized from the cell surface (Figure 2, J–L). These data indicate that the IL-2R-VE-cadcyto follows the same pathway for internalization as does endogenous VE-cadherin. These results provide further evidence that the VE-cadherin tail provides targeting information for cadherin endocytosis.
Figure 2.
IL-2R-VE-cadcyto follows the same internalization pathway as endogenous VE-cadherin. Cell surface VE-cadherin in untreated MECs was labeled by an Alexa Fluor 555-conjugated mAb (BV6) directed against the extracellular domain of VE-cadherin at 4°C. Cells were then transferred to 37°C for 15 min. After acid washing to remove the antibodies bound to the cell surface, the localization of internalized VE-cadherin was examined by immunofluorescence microscopy and compared with EEA-1 (A–C) and transferrin (D–F) localization. The colocalization of internalized IL-2R-VE-cadcyto and EEA-1 was also determined (G–I). The IL-2R-VE-cadcyto was monitored by using an Alexa Fluor 555-conjugated IL-2R antibody. Furthermore, the colocalization of internalized IL-2R-VE-cadcyto and endogenous VE-cadherin was examined in MECs expressing IL-2R-VE-cadcyto. The IL-2R-VE-cadcyto was followed by using an Alexa Fluor 488-conjugated IL-2R antibody and the endogenous VE-cadherin was followed by Alexa Fluor 555-conjugated BV6 VE-cadherin antibody directed against the extracellular domain of VE-cadherin (J–L). Bar, 50 μm.
VE-Cadherin Internalization Is Clathrin Dependent
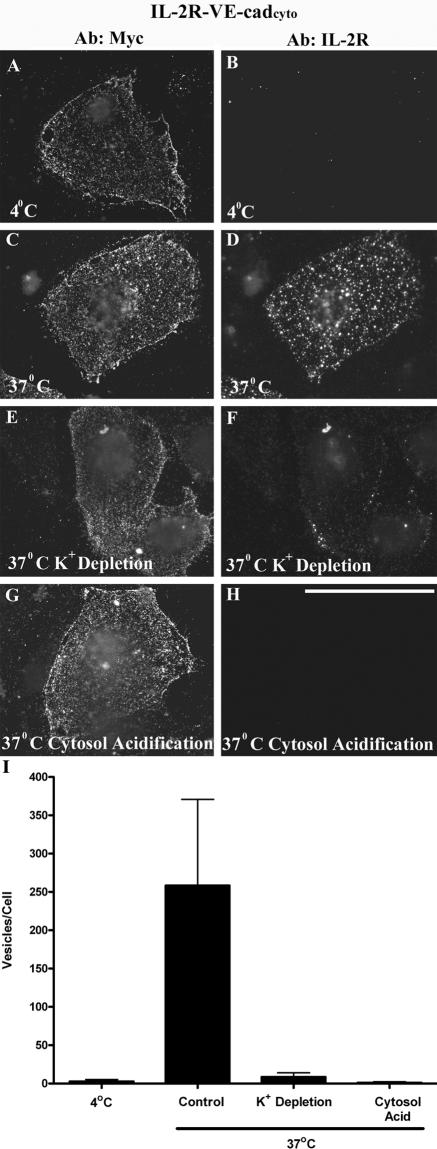
Recent studies indicate that multiple pathways may mediate E-cadherin internalization in various cell types, including clathrin-dependent and clathrin-independent mechanisms (Akhtar and Hotchin, 2001; Palacios et al., 2002; Paterson et al., 2003; Bryant and Stow, 2004). Although multiple pathways for internalization can converge on transferrin-positive compartments, the colocalization between VE-cadherin and transferrin in endocytic compartments (Figure 2, D–E) suggested that VE-cadherin may be internalized via a clathrin mediated pathway. To test this possibility directly, clathrin-dependent endocytosis was inhibited using K+ depletion and cytosol acidification, two well-characterized methods to inhibit clathrin-mediated internalization (Wu et al., 2001; Salazar and Gonzalez, 2002). Endocytosis mediated by the VE-cadherin cytoplasmic domain was monitored by expressing the IL-2R-VE-cadcyto chimeric protein in MEC as shown in Figure 1. A myc epitope at the carboxy-terminal tail of the VE-cadherin cytoplasmic domain was used to verify expression of the IL-2R-VE-cadcyto chimera. Similar to the results shown in Figure 1, extensive internalization of the IL-2R-VE-cadcyto chimera was observed after 5 min at 37°C (Figure 3D). Both K+ depletion (Figure 3, E and F) and cytosol acidification (Figure 3, G and H) dramatically inhibited internalization of the IL-2R-VE-cadcyto polypeptide. These results, shown quantitatively in Figure 3I, indicate that endocytosis of the IL-2R-VE-cadcyto chimera is clathrin dependent.
Figure 3.
IL-2R-VE-cadcyto internalization is clathrin dependent. Cell surface IL-2R-VE-cadcyto was labeled using the IL-2R antibody at 4°C. Cells were then kept at 4°C (A and B) or transferred to 37°C for 5 min (C–H). At the end of the incubation period, cells were processed for immunofluorescence using antibodies directed against the myc epitope tag at the carboxy-terminal domain of the IL-2R-VE-cadcyto to verify expression of the protein. After 5 min at 37°C, substantial internalization of the IL-2R-VE-cadcyto was observed (C and D). Cytosol acidification (E and F) and potassium depletion (G and H), both of which inhibit clathrin mediated endocytosis, completely blocked internalization of the IL-2R-VE-cadcyto chimera (I). Bar, 50 μm.
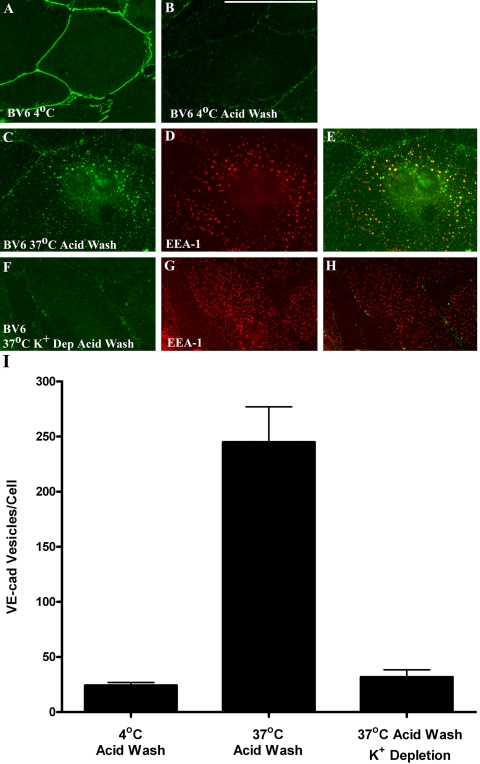
Additional experiments were performed to verify that endogenous VE-cadherin is internalized via mechanisms similar to the IL-2R-VE-cadcyto polypeptide. For these experiments, cell surface VE-cadherin was followed using a fluorescently tagged mAb (BV6) directed against the VE-cadherin extracellular domain. In cells incubated at 4°C, BV6 decorated cell-cell junctions as well as nonjunctional cell surface VE-cadherin (Figure 4A). Cell surface BV6 labeling was efficiently removed upon acid washing cells incubated at 4°C (Figure 4B). After 15 min at 37°C, VE-cadherin was internalized and targeted to early endosomes as assessed by EEA-1 localization (Figure 4, C–E). Both K+ depletion (Figure 4, F–H) and cytosol acidification (our unpublished data) dramatically blocked endocytosis of endogenous VE-cadherin (Figure 4I). Together with the results shown in Figure 3, these data indicate that the VE-cadherin cytoplasmic domain mediates internalization via clathrin-dependent mechanisms.
Figure 4.
VE-cadherin internalization is clathrin dependent. Cell surface VE-cadherin was labeled using BV6 VE-cadherin antibody conjugated to Alexa Fluor 555. Localization of VE-cadherin was examined by immunofluorescence microscopy in cells incubated at 4°C (A and B) or transferred to 37°C for 15 min (C–H). Acid washing removed virtually all surface bound BV6 (B). Colocalization with EEA-1 indicated that the internalized VE-cadherin was targeted to early endosomes (C–E). K+ depletion dramatically blocked endocytosis of endogenous VE-cadherin (F–H), indicating that VE-cadherin internalization was clathrin-mediated. Results from the immunofluorescence experiments are shown quantitatively in I. Bar, 50 μm.
p120 Selectively Inhibits VE-Cadherin Endocytosis
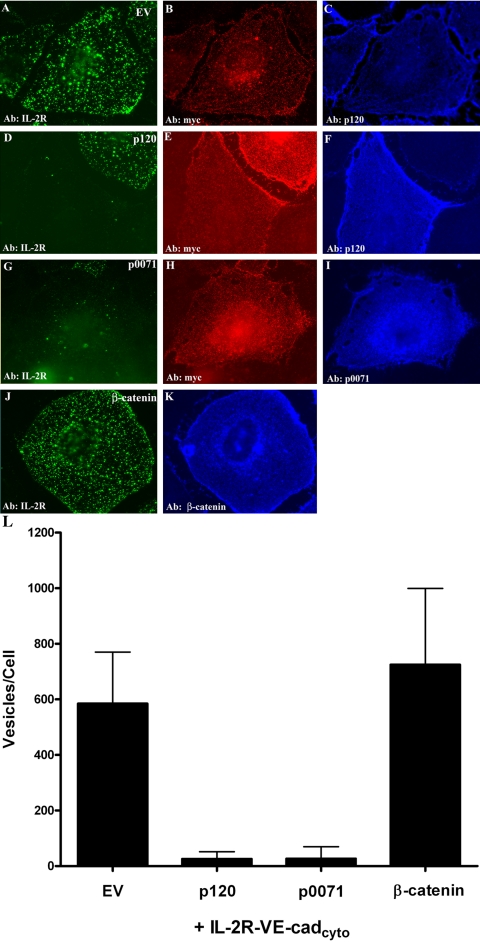
In previous studies, p120 was found to inhibit the delivery of cell surface derived VE-cadherin to lysosomes (Xiao et al., 2003a). However, these experiments lacked the temporal resolution required to determine whether p120 specifically inhibited endocytosis of the cadherin. The results shown above indicate that the IL-2R-VE-cadcyto chimera is internalized in a clathrin-dependent manner and routed to identical compartments as endogenous VE-cadherin. Furthermore, because the IL-2R-VE-cadcyto polypeptide is not engaged in adhesive interactions, significant amounts of the IL-2R-VE-cadcyto chimera can be detected in intracellular pools within 5 min after switching cells to 37°C (Izumi et al., 2004). Therefore, we tested the ability of p120 to inhibit endocytosis of the IL-2R-VE-cadcyto chimera using short time courses (5 min) to determine whether p120 regulates early steps in cadherin internalization. For these experiments, IL-2R-VE-cadcyto chimera was expressed with empty vector or in combination with p120 using an adenoviral delivery system. In the absence of exogenous p120, substantial levels of IL-2R-VE-cadcyto chimera internalization were observed within 5 min (Figure 5, A–C). In contrast, endocytosis of the IL-2R-VE-cadcyto polypeptide was virtually eliminated in cells coexpressing exogenous p120 (Figure 5, D–F). Interestingly, the p120 related protein p0071 also inhibited VE-cadherin endocytosis (Figure 5, G–I). However, β-catenin was unable to inhibit internalization (Figure 5, J and K). These experiments demonstrate that p120 family proteins specifically and potently inhibit VE-cadherin endocytosis.
Figure 5.
p120 prevents endocytosis of VE-cadherin. The IL-2R-VE-cadcyto chimera was expressed with empty virus (A–C) or in combination with p120 (D–F), p0071 (G–I), or β-catenin (J and K). Cell surface IL-2R-VE-cadcyto was labeled using IL-2R antibody at 4°C, and then cells were transferred to 37°C for 5 min. After acid washing, localization of internalized IL-2R-VE-cadcyto was examined by immunofluorescence microscopy. Expression of the IL-2R-VE-cadcyto polypeptide was verified by using a chicken antibody directed against the myc epitope at the carboxyl terminal tail of the IL-2R-VE-cadherin chimera (B, E, and H). Results from the immunofluorescence experiments are shown quantitatively in L. Bar, 50 μm.
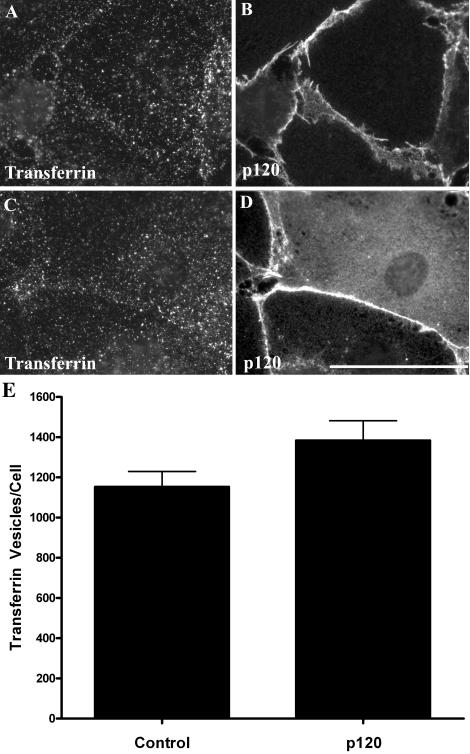
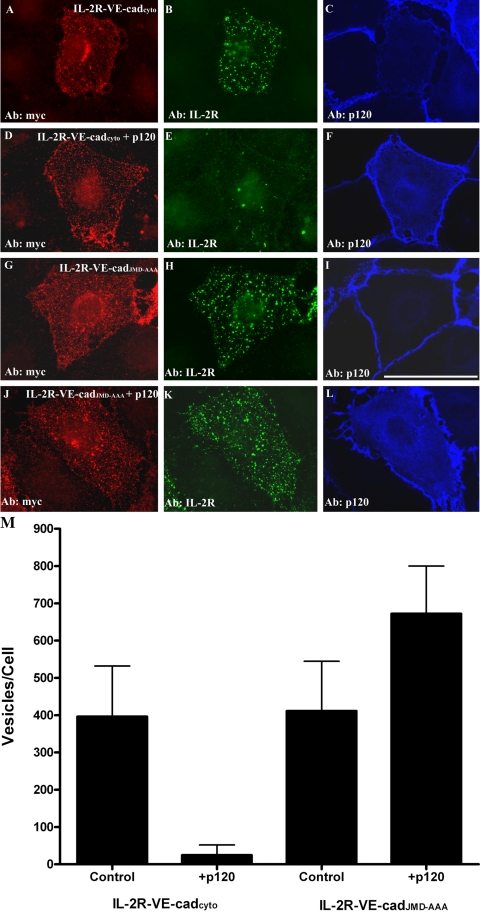
It is possible that p120 inhibits VE-cadherin endocytosis selectively, or alternatively, that p120 functions as a general inhibitor of clathrin-mediated internalization from the plasma membrane. To distinguish these possibilities, two approaches were taken. First, the ability of p120 to block endocytosis of a prototypical clathrin cargo protein was tested. For these experiments, the effect of p120 on transferrin endocytosis was tested. As shown in Figure 6, transferrin internalization was quantitatively similar in control endothelial cells and in endothelial cells overexpressing p120. These findings suggest that p120 does not globally alter the kinetics of clathrin-mediated endocytic pathways. Based on these results, we tested whether p120 binding to the VE-cadherin tail was required for p120 to inhibit VE-cadherin endocytosis. In previous studies, a triple amino-acid substitution in the cadherin juxtamembrane domain was shown to abrogate p120 binding to the cadherin tail (Thoreson et al., 2000; Calkins et al., 2003; Xiao et al., 2003a). Therefore, this mutation was introduced into the VE-cadherin cytoplasmic domain in the context of the IL-2R-VE-cadcyto chimera. The resulting polypeptide, termed IL-2R-VE-cadJMD-AAA, encodes the VE-cadherin cytoplasmic tail with a triple alanine substitution at amino acids 562–564 (EMD-AAA), which abrogates p120 and p0071 binding (Calkins et al., 2003). As shown in Figure 7, p120 dramatically decreased endocytosis of IL-2R-VE-cadcyto chimera (Figure 7, D–F). However, the IL-2R-VE-cadJMD-AAA construct was completely refractile to overexpression of p120; even in cells expressing high levels of p120, substantial internalization of the IL-2R-VE-cadJMD-AAA polypeptide was observed (Figure 7, J–L). These data indicate that binding of p120 to the cadherin tail is required for p120 to inhibit VE-cadherin endocytosis. Furthermore, that p120 does not prevent transferrin internalization indicates that p120 regulates VE-cadherin endocytosis selectively rather than clathrin-dependent endocytosis globally.
Figure 6.
p120 does not inhibit transferrin receptor internalization. MECs infected with empty virus or virus carrying p120 were incubated with Alexa Fluor 555-conjugated transferrin at 4°C and then transferred to 37°C for 5 min. After acid washing, cells were fixed and processed for immunofluorescence microscopy. The localization of internalized transferrin was examined in both control MEC cells (A and B) and MECs expressing p120 (C and D). Results are shown quantitatively in E. Bar, 50 μm.
Figure 7.
p120 binding to the VE-cadherin tail is required for inhibition of endocytosis. p120 was coexpressed in MEC expressing either IL-2R-VE-cadcyto or the IL-2R-VE-cadJMD-AAA, which does not bind p120. Cell surface IL-2R-VE-cadherin polypeptides were labeled using the IL-2R antibody at 4°C and then transferred to 37°C for 5 min. After acid washing, cells were fixed and processed for triple-labeled immunofluorescence microscopy. Internalized IL-2R-VE-cadherin polypeptides were detected using an Alexa Fluor 555 goat anti-mouse secondary antibody (B, E, H, and K). Expression of the IL-2R-VE-cadcyto chimera was verified by using a chicken antibody directed against the myc epitope at the carboxy-terminal tail of the IL-2R-VE-cadherin polypeptide (A, D, G, and J). Expression of p120 in the cells was verified using a rabbit p120 antibody (C, F, I, and L). Results from the immunofluorescence experiments are shown quantitatively in M. Bar, 50 μm.
p120 Dissociates from VE-Cadherin upon Internalization
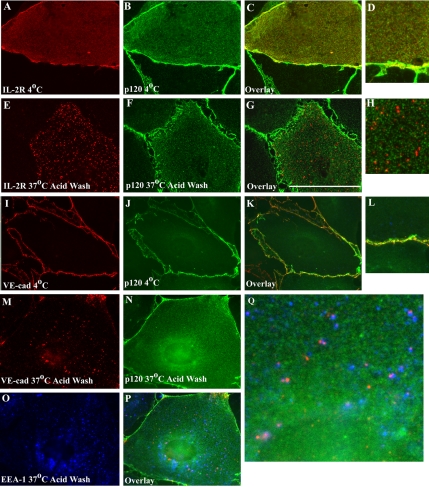
The aforementioned data suggest that p120 binding to the VE-cadherin at the plasma membrane stabilizes the cadherin and prevents internalization. A prediction based on these observations is that p120 would dissociate from the cadherin during internalization. In previous studies, p120 did not colocalize with vesicular pools of VE-cadherin in chloroquine-treated MECs. However, these previous internalization assays were carried out over long periods (3–6 h), allowing substantial amounts of time for p120 to dissociate after endocytosis. To investigate whether p120 is associated with the VE-cadherin tail upon and immediately after endocytosis, p120 localization was monitored relative to the IL-2R-VE-cadcyto chimera. In cells that remained at 4°C, the IL-2R-VE-cadcyto chimera colocalized extensively with endogenous p120 at the cell surface (Figure 8, A–D). In contrast, 5 min after internalization at 37°C, endogenous p120 failed to colocalize with the internalized IL-2R-VE-cadcyto polypeptide, suggesting that p120 dissociated from the IL-2R-VE-cadcyto polypeptide upon internalization (Figure 8, E–H). Similar results were obtained when β-catenin localization was examined (our unpublished data), suggesting that both catenins may dissociate from the VE-cadherin cytoplasmic tail upon endocytosis. A caveat to these experiments is that epitope masking of p120 could occur upon entry of the complex into endosomes. Therefore, internalization of endogenous VE-cadherin was followed using the fluorescently tagged BV6 mAb in MECs expressing low levels of GFP-tagged p120. The p120.GFP assembled into junctions and exhibited extensive colocalization with BV6 at the plasma membrane (Figure 8, I–L). However, p120.GFP did not colocalize in early endosomes with the BV6-VE-cadherin complex derived from the cell surface (Figure 8, M–Q). These data support a model in which p120 dissociates from the VE-cadherin tail upon clathrin-mediated internalization of the cadherin from the plasma membrane.
Figure 8.
p120 dissociates from VE-cadherin upon internalization. MEC cells were infected with adenovirus carrying the IL-2R-VE-cadcyto chimera and localization of endogenous p120 was monitored relative to the IL-2R-VE-cadcyto chimera. At 4°C, the IL-2R-VE-cadcyto polypeptide colocalized extensively with endogenous p120 at the cell surface (A–D). However, 5 min after internalization at 37°C, endogenous p120 failed to colocalize with the internalized IL-2R-VE-cadcyto (E–H). To further test if p120 dissociates from VE-cadherin upon internalization, internalization of endogenous VE-cadherin was followed using the Alexa Fluor 555-tagged BV6 mAb in MEC expressing GFP-tagged p120. The p120.GFP assembled into junctions and exhibited extensive colocalization with the BV6-VE-cadherin complex at the plasma membrane at 4°C (I–L). However, 30 min after internalization at 37°C, p120.GFP did not colocalize with BV6–VE-cadherin complexes that had been internalized to early endosomes (M–Q). Bar, 50 μm.
DISCUSSION
The work presented here demonstrates that the cytoplasmic tail of VE-cadherin targets the protein for internalization via a clathrin-dependent pathway. Furthermore, p120 selectively prevents clathrin-dependent endocytic machinery from targeting the cadherin for endocytosis. These findings establish a novel mechanism whereby a cytoplasmic binding partner functions as a specific plasma membrane retention signal for a transmembrane receptor. In addition, the results presented here clarify the mechanism by which p120 functions as a set point to regulate cadherin cell surface expression levels.
Previous studies in several model systems demonstrated that p120 catenin functions as a set point, or rheostat, to control cadherin expression levels in vascular endothelial cells and in epithelial cells (Davis et al., 2003; Xiao et al., 2003a; Iyer et al., 2004). Furthermore, E-cadherin is metabolically unstable in tumor cell lines lacking p120, suggesting that loss of p120 leads to a corresponding loss of E-cadherin during tumor progression (Ireton et al., 2002; Reynolds and Carnahan, 2004). Collectively, these findings revealed a core function for p120 in the stabilization of cell surface cadherins. However, the mechanism by which p120 controls the metabolic stability of cadherins was not clear from these previous studies. Newly synthesized E-cadherin is delivered to the plasma membrane efficiently in the absence of p120, suggesting that p120 is dispensable in cadherin trafficking to the plasma membrane after translation (Davis et al., 2003). Similarly, the plasma membrane pool of VE-cadherin becomes destabilized in the absence of p120, leading to VE-cadherin degradation via a lysosomal pathway (Xiao et al., 2003a). However, other studies found that p120 binds to kinesin and that this interaction modulates the rate of N-cadherin accumulation at cell-cell borders upon initiation of cell contact in response to calcium (Chen et al., 2003). Although these previous studies clearly established a role for p120 in cadherin trafficking and metabolic stability, the experiments lacked the temporal resolution necessary to implicate p120 in any particular step of membrane trafficking.
In the present study, we used several approaches to identify the cellular machinery involved in VE-cadherin internalization and to directly test whether p120 regulates this step in cadherin trafficking. First, a gain of function approach was used to establish that the VE-cadherin cytoplasmic tail harbors the information necessary to target the cadherin for internalization. Indeed, fusion of the VE-cadherin tail to the IL-2R dramatically enhanced the baseline rate of IL-2R internalization (Figure 1). In addition, internalization of both the IL-2R-VE-cadcyto chimera and endogenous VE-cadherin were mediated by a clathrin-dependent pathway (Figures 3 and 4). Interestingly, cointernalization assays demonstrated extensive colocalization between the IL-2R-VE-cadcyto chimera and endogenous VE-cadherin (Figure 2). These data demonstrate that the tail of the cadherin is sufficient not only to mediate clathrin-dependent endocytosis but also to target the cadherin to specific subcellular compartments after endocytosis.
The IL-2R-VE-cadcyto chimera is unable to engage in adhesion, and the protein is rapidly internalized from the cell surface. Substantial levels of internalized IL-2R-VE-cadcyto were detected within 5 min, as assessed by both fluorescence-based antibody internalization assays as well as cell surface biotinylation experiments. These findings are consistent with the idea that engagement of adhesion prevents cadherin endocytosis (Izumi et al., 2004). The rapid kinetics of endocytosis of this protein allowed us to determine whether p120 specifically regulates VE-cadherin internalization and thus to isolate this step in the trafficking pathway from temporally subsequent steps in sorting, such as recycling and degradation. The results indicate that p120 dramatically inhibits cadherin endocytosis (Figure 5). Furthermore, mutation of the p120 binding site on the cadherin relieves this inhibition (Figure 7). These data indicate that p120 must bind to the cadherin to function as a plasma membrane retention signal. Interestingly, p120 had no discernible impact on transferrin internalization (Figure 6), indicating that the p120 effect is selective for cadherins and not the endocytic pathway globally. These data reveal a novel mechanism whereby a cytoplasmic binding partner can function as a highly selective plasma membrane retention signal for a transmembrane binding partner. It is formally possible that in addition to regulating the endocytosis of cadherins, p120 may also regulate subsequent steps in cadherin trafficking, such as recycling (Le et al., 1999; Mary et al., 2002; Bryant and Stow, 2004). Our data do not rule out this possibility. As discussed above, previous work demonstrated that p120 associates with kinesin and that this interaction plays a role in the movement of vesicular pools of N-cadherin out toward intercellular junctions (Chen et al., 2003). These data suggest that p120 may play a role in recycling of the cadherin after endocytosis. However, the effects of p120 on junctional accumulation of N-cadherin were quantitatively modest compared with the effects of p120 on VE-cadherin internalization. In addition, although the rates of movement of N-cadherin toward the plasma membrane are slower if the p120 binding site on the cadherin is ablated, mutant N-cadherin that is unable to bind p120 is still delivered to intercellular junctions (Chen et al., 2003). Finally, newly synthesized E-cadherin does not seem at all dependent upon p120 for proper delivery to the plasma membrane (Davis et al., 2003). Thus, although p120 may have some effects on cadherin recycling, the data presented here indicate that quantitatively the most important point of cadherin regulation by p120 is an early step in cadherin internalization from the plasma membrane.
The model that emerges from these studies is that the VE-cadherin tail associates with cellular machinery involved in clathrin-dependent endocytosis and that p120 somehow prevents these interactions. Several sequence motifs in the VE-cadherin tail exhibit homology to di-leucine- or tyrosine-based internalization signals. These amino acid motifs mediate binding to adaptor complexes, such as AP-2, and thereby couple cargo molecules to clathrin-coated pits (Bonifacino and Traub, 2003; Robinson, 2004). An attractive possibility is that p120 competes for such interactions and thereby functions to prevent entry of the cadherin into the endocytic pathway. This possibility is supported by the observation that p120 colocalizes strongly with junctional VE-cadherin, but it is not present in vesicular pools of VE-cadherin that have been internalized from the plasma membrane. Nonetheless, it is clear that p120 also exhibits influence over small GTPases and actin cytoskeletal organization (Anastasiadis and Reynolds, 2001), thus raising the possibility that a more indirect mechanism of action may underlie p120 regulation of cadherin internalization. However, the fact that p120 must bind to the cadherin leads us to favor a model in which p120 binding to the cadherin functions to “cap” the cadherin tail, thereby preventing adaptor protein interactions and subsequent endocytosis (Figure 9). Studies are underway to distinguish between these possibilities and to identify the function of this regulatory system in the context of endothelial cell biology in vivo. Indeed, recent studies from the Radice laboratory indicate that VE-cadherin expression levels are regulated by N-cadherin, possibly through the regulation of p120 levels (Luo and Radice, 2005). These data suggest that p120, and perhaps other classical cadherins, exert influence over VE-cadherin expression levels during mouse vascular development.
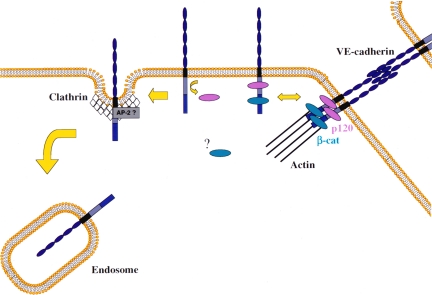
Figure 9.
p120 regulates clathrin-dependent endocytosis of VE-cadherin. Similar to other classical cadherins, VE-cadherin associates with the catenins and assembles into intercellular junctions. A pool of VE-cadherin may also be present outside of the junctions at the plasma membrane. On endocytosis, p120 dissociates from the VE-cadherin tail. The loss of p120 is predicted to expose sequence motifs on the cadherin cytoplasmic domain that mediate association with adaptor proteins involved in clathrin-dependent internalization, such as AP-2. Regardless of the precise mechanism, p120 functions to regulate plasma membrane levels of cadherins by regulating internalization from the plasma membrane. The model reveals a unique molecular mechanism by which a cytoplasmic protein, in this case p120, selectively regulates clathrin mediated endocytosis of a transmembrane receptor.
Acknowledgments
We are grateful to Dr. Kathleen Green for insightful comments and advice and to Dr. Gloria Salazar for assistance with the clathrin-dependent endocytosis assays. Additional thanks go to members of the Kowalczyk laboratory for helpful advice and discussion. This work was supported by National Institutes of Health Grants R01 AR050501 and R01 AR48266 (to A.P.K.). K. X. was supported by a postdoctoral fellowship from the American Heart Association.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–05–0440) on August 24, 2005.
Abbreviations used: IL-2R, interleukin 2 receptor; MEC, microvascular endothelial cell; p120: p120-catenin.
References
- Akhtar, N., and Hotchin, N. A. (2001). RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol. Biol. Cell 12, 847–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis, P. Z., and Reynolds, A. B. (2000). The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113, 1319–1334. [DOI] [PubMed] [Google Scholar]
- Anastasiadis, P. Z., and Reynolds, A. B. (2001). Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13, 604–610. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J. S., and Traub, L. M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395–447. [DOI] [PubMed] [Google Scholar]
- Breviario, F., Caveda, L., Corada, M., Martin-Padura, I., Navarro, P., Golay, J., Introna, M., Gulino, D., Lampugnani, M. G., Dejana, E. (1995). Functional properties of human vascular endothelial cadherin (7B4/Cadherin-5), an endothelium-specific cadherin. Arterioscler. Thromb. Vasc. Biol. 15, 1229–1239. [DOI] [PubMed] [Google Scholar]
- Bryant, D. M., and Stow, J. L. (2004). The ins and outs of E-cadherin trafficking. Trends Cell Biol. 14, 427–434. [DOI] [PubMed] [Google Scholar]
- Calkins, C. C., Hoepner, B. L., Law, C. M., Novak, M. R., Setzer, S. V., Hatzfeld, M., and Kowalczyk, A. P. (2003). The Armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J Biol. Chem. 278, 1774–1783. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P., and Collen, D. (2000). Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann. N.Y. Acad. Sci. 902, 249–262. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P., et al. (1999). Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98, 147–157. [DOI] [PubMed] [Google Scholar]
- Chen, X., Kojima, S. I., Borisy, G. G., and Green, K. J. (2003). p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J. Cell Biol. 163, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada, M., Liao, F., Lindgren, M., Lampugnani, M. G., Breviario, F., Frank, R., Muller, W. A., Hicklin, D. J., Bohlen, P., and Dejana, E. (2001). Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 97, 1679–1684. [DOI] [PubMed] [Google Scholar]
- Corada, M., Zanetta, L., Orsenigo, F., Breviario, F., Lampugnani, M. G., Bernasconi, S., Liao, F., Hicklin, D. J., Bohlen, P., and Dejana, E. (2002). A mAb to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood 100, 905–911. [DOI] [PubMed] [Google Scholar]
- Crosby, C. V., Fleming, P. A., Argraves, W. S., Corada, M., Zanetta, L., Dejana, E., and Drake, C. J. (2005). VE-cadherin is not required for the fort-nation of nascent blood vessels but acts to prevent their disassembly. Blood 105, 2771–2776. [DOI] [PubMed] [Google Scholar]
- Davis, M. A., Ireton, R. C., and Reynolds, A. B. (2003). A core function for p120-catenin in cadherin turnover. J. Cell Biol. 163, 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana, E. (2004). Endothelial cell-cell junctions: happy together. Nat. Rev. Mol. Cell Biol. 5, 261–270. [DOI] [PubMed] [Google Scholar]
- Dejana, E., Spagnuolo, R., and Bazzoni, G. (2001). Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb. Haemost. 86, 308–315. [PubMed] [Google Scholar]
- Faundez, V., Horng, J. T., and Kelly, R. B. (1997). ADP ribosylation factor 1 is required for synaptic vesicle budding in PC12 cells. J. Cell Biol. 138, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicano, G. I., Bauer, C., and Fuchs, E. (2001). Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development 128, 929–941. [DOI] [PubMed] [Google Scholar]
- Gooding, J. M., Yap, K. L., and Ikura, M. (2004). The cadherin-catenin complex as a focal point of cell adhesion and signalling: new insights from three-dimensional structures. Bioessays 26, 497–511. [DOI] [PubMed] [Google Scholar]
- Hammond, D. E., Carter, S., McCullough, J., Urbe, S., Vande Woude, G., and Clague, M. J. (2003). Endosomal dynamics of Met determine signaling output. Mol. Biol. Cell 14, 1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld, M. (2005). The p120 family of cell adhesion molecules. Eur. J. Cell Biol. 84, 205–214. [DOI] [PubMed] [Google Scholar]
- He, T. C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W., and Vogelstein, B. (1998). A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton, R. C., et al. (2002). A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 159, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, S., Ferreri, D. M., DeCocco, N. C., Minnear, F. L., and Vincent, P. A. (2004). VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am. J. Physiol. 286, L1143–L1153. [DOI] [PubMed] [Google Scholar]
- Izumi, G., Sakisaka, T., Baba, T., Tanaka, S., Morimoto, K., and Takai, Y. (2004). Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J. Cell Biol. 166, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk, A. P., Navarro, P., Dejana, E., Bornslaeger, E. A., Green, K. J., Kopp, D. S., Borgwardt, J. E. (1998). VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J. Cell Sci. 111, 3045–3057. [DOI] [PubMed] [Google Scholar]
- Kowalczyk, A. P., and Reynolds, A. B. (2004). Protecting your tail: regulation of cadherin degradation by p120-catenin. Curr. Opin. Cell Biol. 16, 522–527. [DOI] [PubMed] [Google Scholar]
- Lampugnani, M. G., et al. (2003). Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 161, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, T. L., Yap, A. S., and Stow, J. L. (1999). Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146, 219–232. [PMC free article] [PubMed] [Google Scholar]
- Liao, F., et al. (2000). mAb to vascular endothelial-cadherin is a potent inhibitor of angiogenesis, tumor growth, and metastasis. Cancer Res. 60, 6805–6810. [PubMed] [Google Scholar]
- Luo, Y., and Radice, G. L. (2005). N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J. Cell Biol. 169, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary, S., Charrasse, S., Meriane, M., Comunale, F., Travo, P., Blangy, A., and Gauthier-Rouviere, C. (2002). Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol. Biol. Cell 13, 285–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, C., et al. (2005). Identification of a transiently exposed VE-cadherin epitope that allows for specific targeting of an antibody to the tumor neovasculature. Blood 105, 4337–4344. [DOI] [PubMed] [Google Scholar]
- Navarro, P., Caveda, L., Breviario, F., Mandoteanu, I., Lampugnani, M. G., and Dejana, E. (1995). Catenin-dependent and -independent functions of vascular endothelial cadherin. J. Biol. Chem. 52, 30965–30972. [DOI] [PubMed] [Google Scholar]
- Ozawa, M., and Ohkubo, T. (2001). Tyrosine phosphorylation of p120(ctn) in v-Src transfected L cells depends on its association with E-cadherin and reduces adhesion activity. J. Cell Sci. 114, 503–512. [DOI] [PubMed] [Google Scholar]
- Palacios, F., Schweitzer, J. K., Boshans, R. L., and D'Souza-Schorey, C. (2002). ARF6-GTP recruits Nm23–H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 4, 929–936. [DOI] [PubMed] [Google Scholar]
- Paterson, A. D., Parton, R. G., Ferguson, C., Stow, J. L., and Yap, A. S. (2003). Characterization of E-cadherin endocytosis in isolated MCF-7 and Chinese hamster ovary cells: the initial fate of unbound E-cadherin. J. Biol. Chem. 278, 21050–21057. [DOI] [PubMed] [Google Scholar]
- Pettitt, J., Cox, E. A., Broadbent, I. D., Flett, A., and Hardin, J. (2003). The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J. Cell Biol. 162, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, A. B., and Carnahan, R. H. (2004). Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin. Cell Dev. Biol. 15, 657–663. [DOI] [PubMed] [Google Scholar]
- Robinson, M. S. (2004). Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167–174. [DOI] [PubMed] [Google Scholar]
- Salazar, G., and Gonzalez, A. (2002). Novel mechanism for regulation of epidermal growth factor receptor endocytosis revealed by protein kinase A inhibition. Mol. Biol. Cell 13, 1677–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer, S. V., Calkins, C. C., Garner, J., Summers, S., Green, K. J., and Kowalczyk, A. P. (2004). Comparative analysis of Armadillo family proteins in the regulation of A431 epithelial cell junction assembly, Adhesion and migration. J. Investig. Dermatol. 123, 426–433. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Lippe, R., Christoforidis, S., Gaullier, J. M., Brech, A., Callaghan, J., Toh, B. H., Murphy, C., Zerial, M., and Stenmark, H. (1998). EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Spagnuolo, R., Corada, M., Orsenigo, F., Zanetta, L., Deuschle, U., Sandy, P., Schneider, C., Drake, C. J., Breviario, F., and Dejana, E. (2004). Gas 1 is induced by VE-cadherin and vascular endothelial growth factor and inhibits endothelial cell apoptosis. Blood 103, 3005–3012. [DOI] [PubMed] [Google Scholar]
- Stevens, T., Garcia, J. G., Shasby, D. M., Bhattacharya, J., and Malik, A. B. (2000). Mechanisms regulating endothelial cell barrier function. Am. J. Physiol. 279, L419–L422. [DOI] [PubMed] [Google Scholar]
- Thoreson, M. A., Anastasiadis, P. Z., Daniel, J. M., Ireton, R. C., Wheelock, M. J., Johnson, K. R., Hummingbird, D. K., and Reynolds, A. B. (2000). Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J. Cell Biol. 148, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiron, O., Chevier, V., Usson, Y., Breviario, F., Job, D., and Dejana, E. (1996). Desmoplakin expression and organization at human umbilical vein endothelial cell-cell junctions. J. Cell Sci. 109, 2141–2149. [DOI] [PubMed] [Google Scholar]
- Vincent, P. A., Xiao, K., Buckley, K. M., and Kowalczyk, A. P. (2004). VE-cadherin: adhesion at arm's length. Am. J. Physiol. 286, C987–C997. [DOI] [PubMed] [Google Scholar]
- Vittet, D., Buchou, T., Schweitzer, A., Dejana, E., and Huber, P. (1997). Targetted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc. Natl. Acad. Sci. USA 94, 6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., Zhao, X., Baylor, L., Kaushal, S., Eisenberg, E., and Greene, L. E. (2001). Clathrin exchange during clathrin-mediated endocytosis. J. Cell Biol. 155, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, K., Allison, D. F., Buckley, K. M., Kottke, M. D., Vincent, P. A., Faundez, V., and Kowalczyk, A. P. (2003a). Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J. Cell Biol. 163, 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, K., Allison, D. F., Kottke, M. D., Summers, S., Sorescu, G. P., Faundez, V., and Kowalczyk, A. P. (2003b). Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J. Biol. Chem. 278, 19199–19208. [DOI] [PubMed] [Google Scholar]