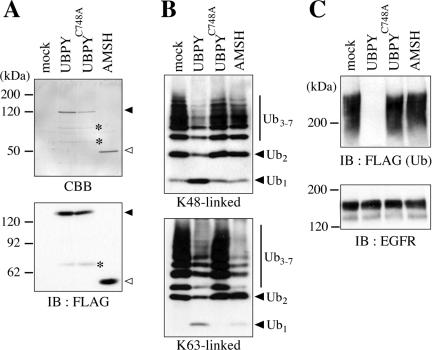
Figure 2.
Deubiquitination of EGFR by UBPY in vitro. (A) FLAG-UBPY, FLAG-UBPYC748A, and FLAG-AMSH were expressed in COS-7 cells, immunoprecipitated with anti-FLAG antibody, and eluted with an excess amount of the FLAG peptide. Proteins in the eluates were separated by SDS-PAGE and detected by CBB staining (top) or immunoblotting with anti-FLAG antibody (bottom). Closed and open arrowheads indicate intact FLAG-UBPY and FLAG-AMSH, respectively. Faint bands designated by asterisks are probably degradation products of UBPY. (B) Immunopurified UBPY, UBPYC748A, and AMSH were incubated with K48-linked (top) or K63-linked (bottom) Ub chains, and the reaction products were detected by immunoblotting with anti-Ub antibody. Positions of the Ub monomer (Ub1), dimer (Ub2), and trimer-heptamer (Ub3–7) are indicated. (C) Immunopurified UBPY, UBPYC748A, and AMSH were incubated with ubiquitinated EGFR that was immunoprecipitated from EGF-stimulated COS-7 cells transfected with FLAG-Ub. The reaction products were detected by immunoblotting with anti-FLAG (top) or anti-EGFR (bottom) antibody.