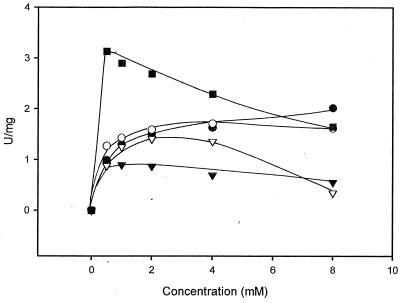
FIG. 2.
Conversion of various concentrations of different amides by enantioselective amidase from R. erythropolis MP50. The reaction mixtures were 0.5 ml and contained 1.4 to 2.8 μg of the purified amidase, 15 μmol of Tris-HCl (pH 7.5), and a 0.5 to 8 mM concentration of 2-methyl-3-phenylpropionamide (•), 2-phenylpropionamide (○), 2-methoxyphenylacetamide (▾), mandeloamide (▿), or 2-chlorophenylacetamide (▪). The reaction mixtures were incubated at room temperature. After 5, 10, 15, 25, and 35 min, aliquots (80 μl each) were taken and the reaction was stopped by the addition of 8 μl of 1 M HCl. The precipitated protein was removed by centrifugation (3 min at 15,800 × g), and the supernatants were reneutralized with 1 M NaOH and analyzed by HPLC for the formation of the respective acids.