Abstract
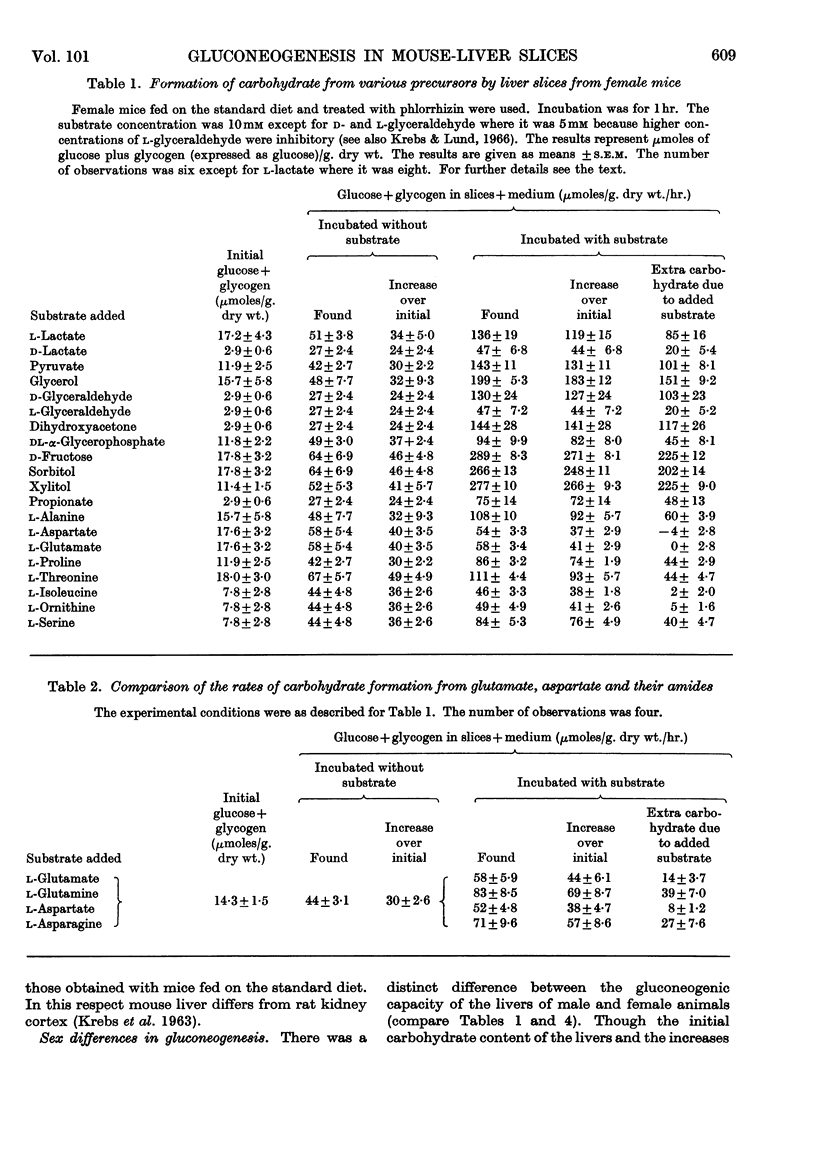
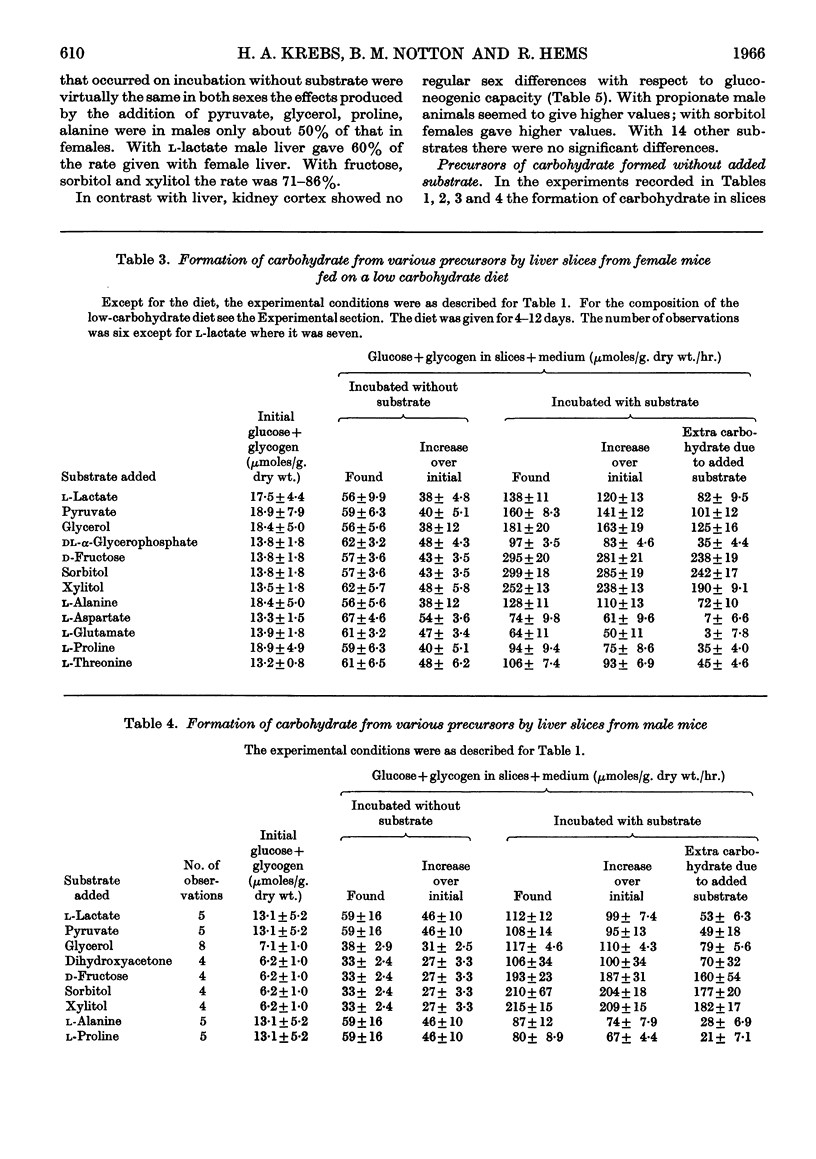
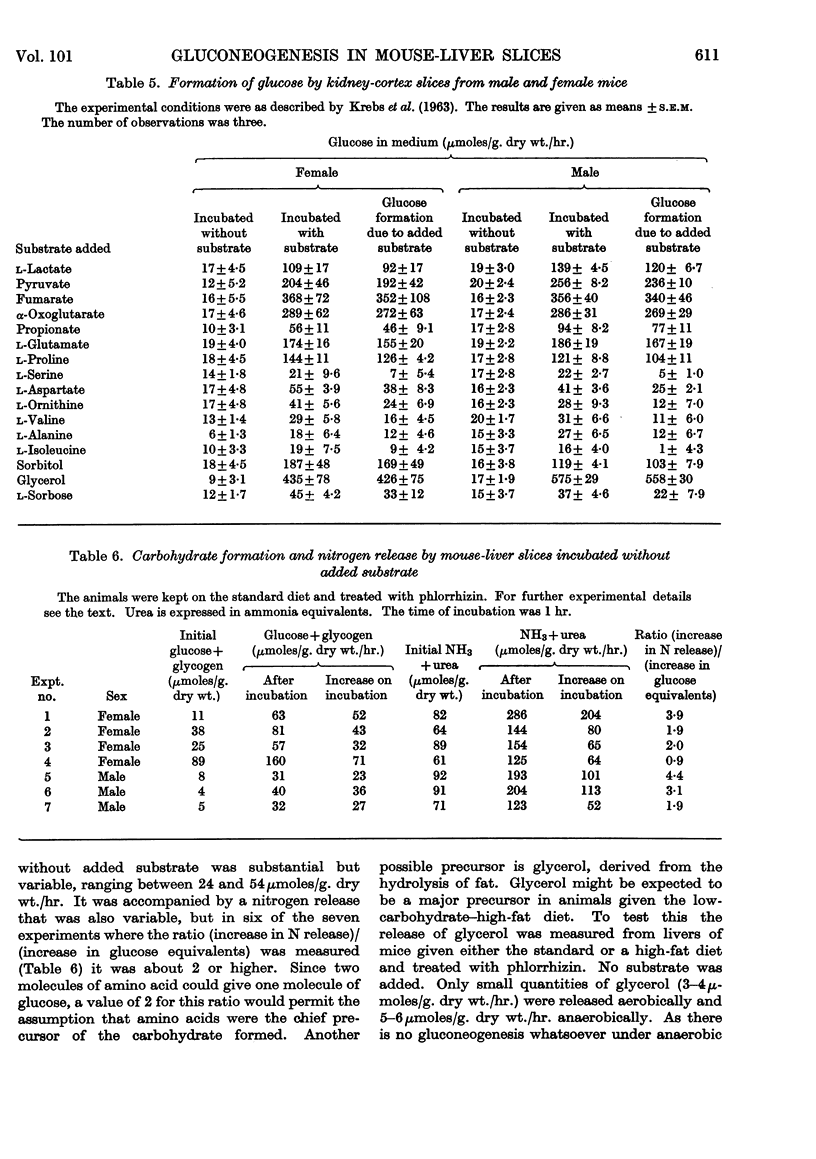
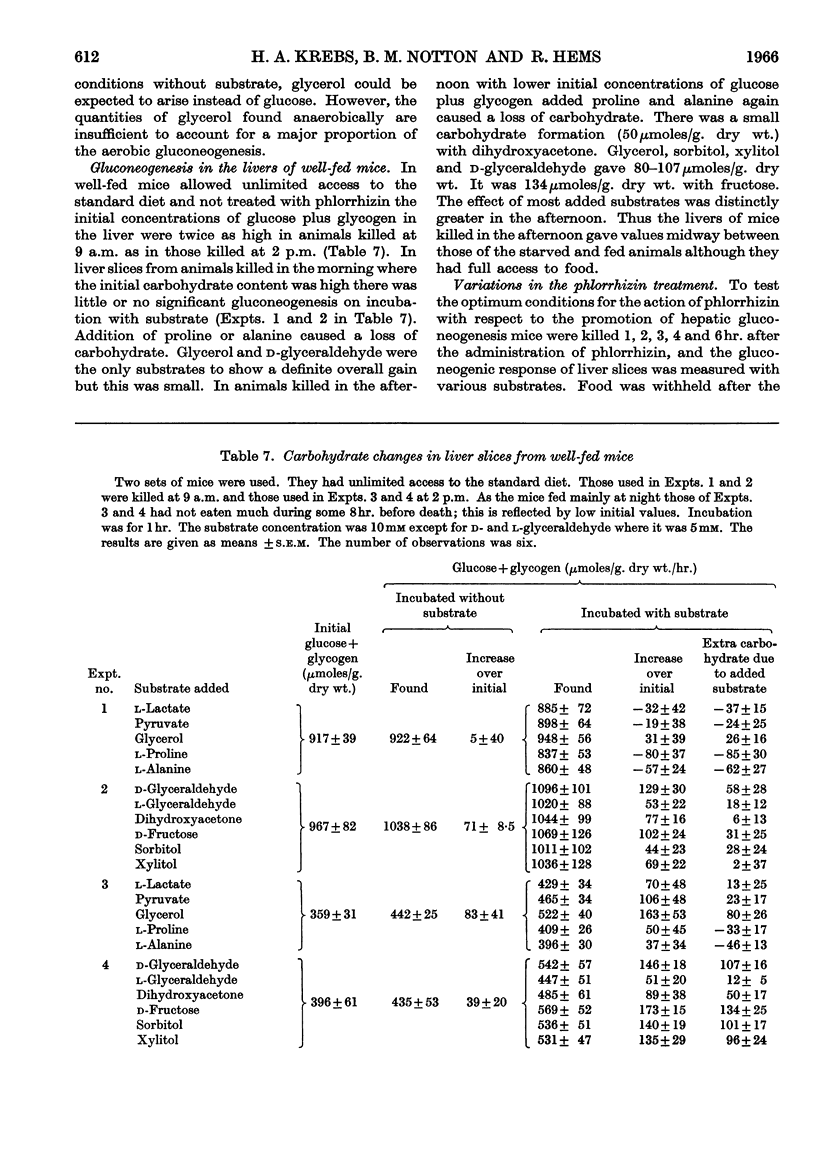
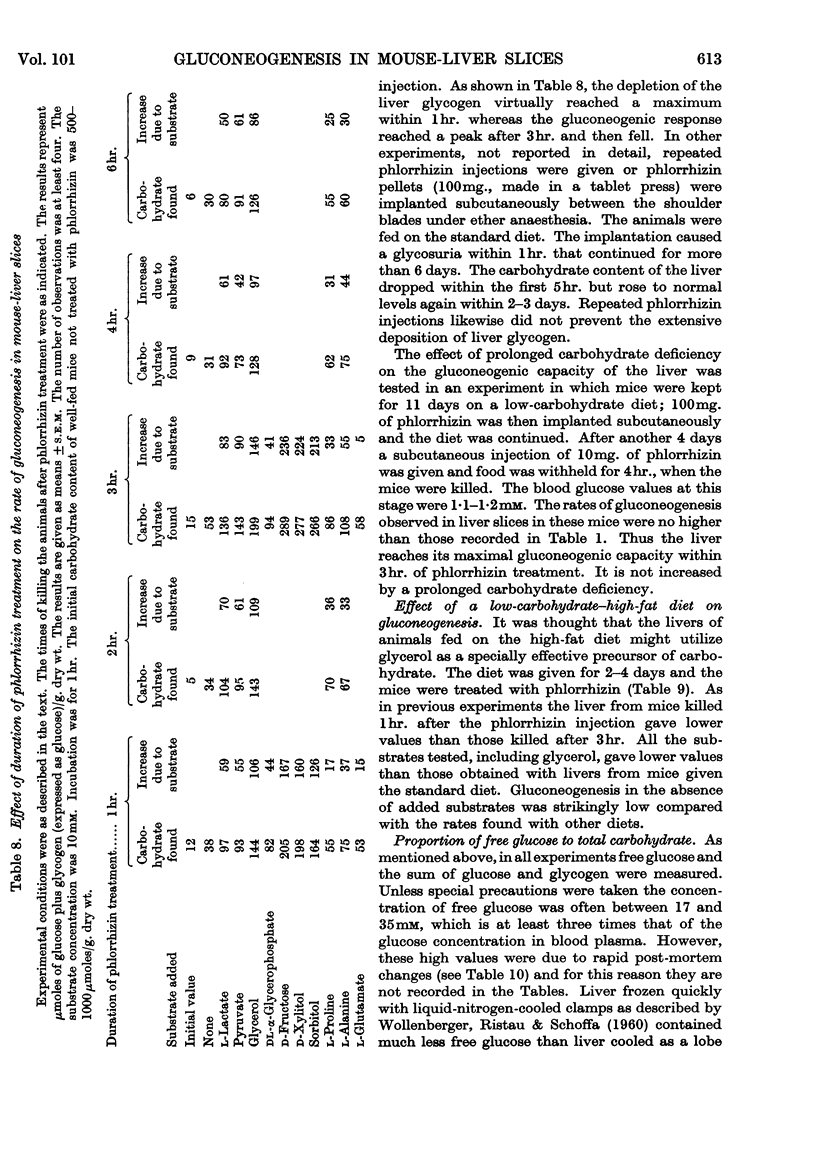
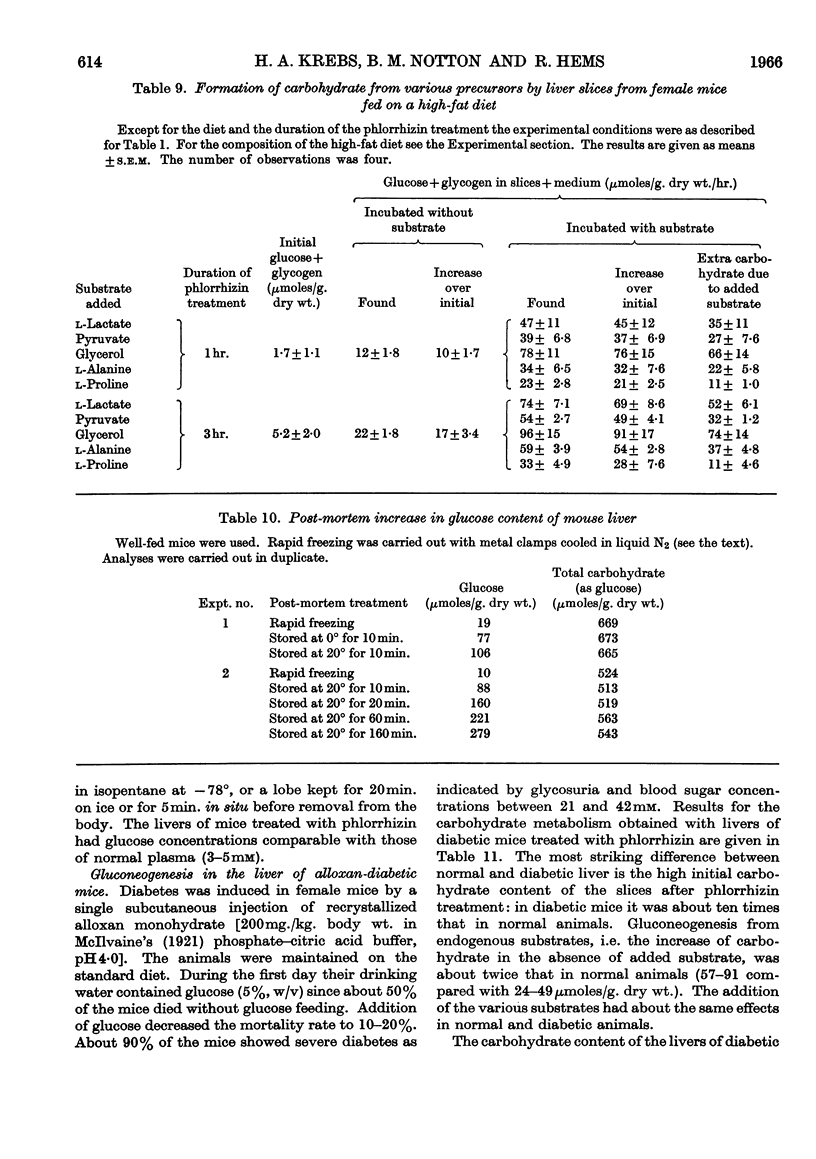
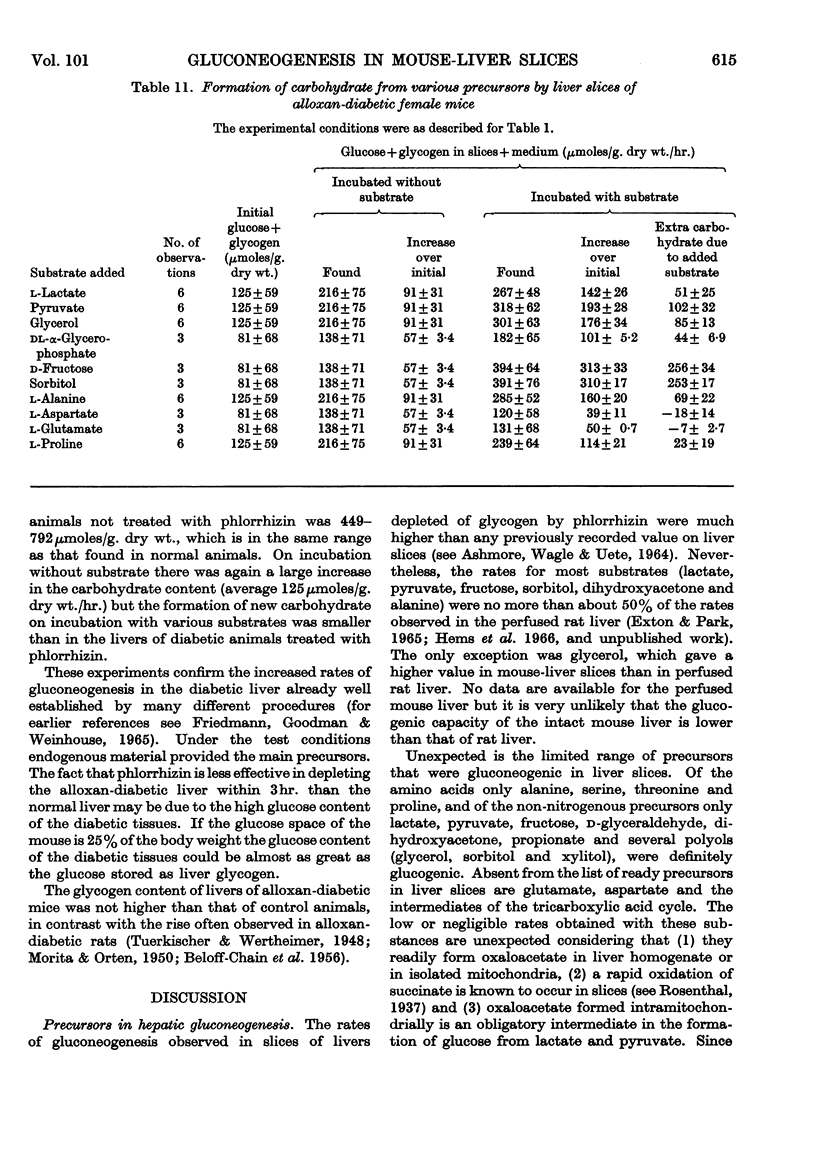
1. The rate of gluconeogenesis from amino acids and other known precursors in slices of mouse liver after depletion of liver glycogen by means of phlorrhizin was high with l-lactate, pyruvate, glycerol, d-glyceraldehyde, dihydroxyacetone, d-fructose, sorbitol, xylitol, α-glycerophosphate, alanine, proline, threonine, serine and propionate. 2. The rate was unexpectedly low or even negligible with glutamate, aspartate, other glucogenic amino acids and the intermediates of the tricarboxylic acid cycle. 3. Glutamine and asparagine gave higher rates than the corresponding amino acids but still much lower rates than kidney cortex. 4. Livers of male mice gave much lower rates than livers of female mice. Kidney cortex showed no sex difference. 5. Livers of mice fed on a low-carbohydrate diet gave the same rates as livers of mice fed on a normal diet under the test conditions, i.e. 3hr. after an injection of phlorrhizin. 6. Much carbohydrate was formed from endogenous precursors and this was accompanied by release of ammonia and urea. 7. Gluconeogenesis in well-fed mice not treated with phlorrhizin was low. 8. Maximum rates were observed 3hr. after phlorrhizin treatment. 9. Prolonged phlorrhizin treatment did not prevent extensive deposition of liver glycogen, after an initial depletion. 10. Gluconeogenesis in livers of mice fed on a high-fat diet was relatively low. 11. Livers of alloxan-diabetic mice had a high carbohydrate content after phlorrhizin treatment, and gluconeogenesis from endogenous sources was about twice as high as in normal animals. Added substrates had about the same effect in normal and diabetic livers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J., Wagle S. R., Uete T. Studies on gluconeogenesis. Adv Enzyme Regul. 1964;2:101–114. doi: 10.1016/s0065-2571(64)80008-x. [DOI] [PubMed] [Google Scholar]
- BROWN L. M., HARRISON M. F. Effect of a single injection of carbon tetrachloride upon the activity of the pseudo-cholinesterase in the liver and serum of male and female rats. Nature. 1951 Jul 14;168(4263):83–84. doi: 10.1038/168083a0. [DOI] [PubMed] [Google Scholar]
- Bach S. J., Holmes E. G. The effect of insulin on carbohydrate formation in the liver. Biochem J. 1937 Jan;31(1):89–100. doi: 10.1042/bj0310089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN W. H., FARMELANT M. H. Effects of androgens and estrogens on beta-glucuronidase in inbred mice. Endocrinology. 1953 May;52(5):536–545. doi: 10.1210/endo-52-5-536. [DOI] [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Dietary and hormonal effects on gluconeogenesis in the rat. J Biol Chem. 1965 Oct;240(10):3729–3735. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaister D., Kerly M. The oxygen consumption and carbohydrate metabolism of the retractor muscle of the foot of Mytilus edulis. J Physiol. 1936 Jun 10;87(1):56–66. doi: 10.1113/jphysiol.1936.sp003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBERMAN H. D., D'ADAMO A. F., Jr Coupling of oxidation of substrates to reductive biosyntheses. III. Studies with L- and D-lactates. J Biol Chem. 1960 Feb;235:514–518. [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. THE CROONIAN LECTURE, 1963. GLUCONEOGENESIS. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:545–564. doi: 10.1098/rspb.1964.0019. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., De Gasquet P. Inhibition of gluconeogenesis by alpha-oxo acids. Biochem J. 1964 Jan;90(1):149–154. doi: 10.1042/bj0900149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Dierks C., Gascoyne T. Carbohydrate synthesis from lactate in pigeon-liver homogenate. Biochem J. 1964 Oct;93(1):112–121. doi: 10.1042/bj0930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Lund P. Formation of glucose from hexoses, pentoses, polyols and related substances in kidney cortex. Biochem J. 1966 Jan;98(1):210–214. doi: 10.1042/bj0980210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal O. The intensity of succinate oxidation in surviving liver tissue. Biochem J. 1937 Oct;31(10):1710–1718. doi: 10.1042/bj0311710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]


