Abstract
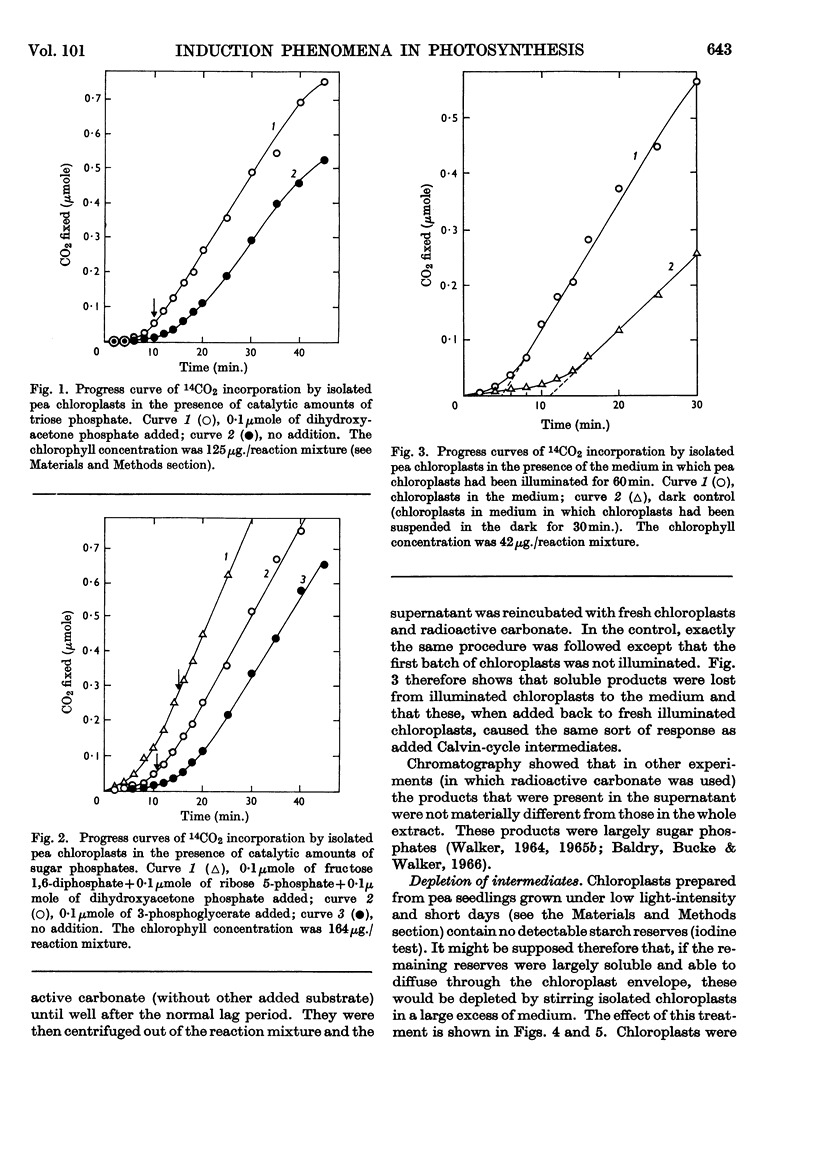
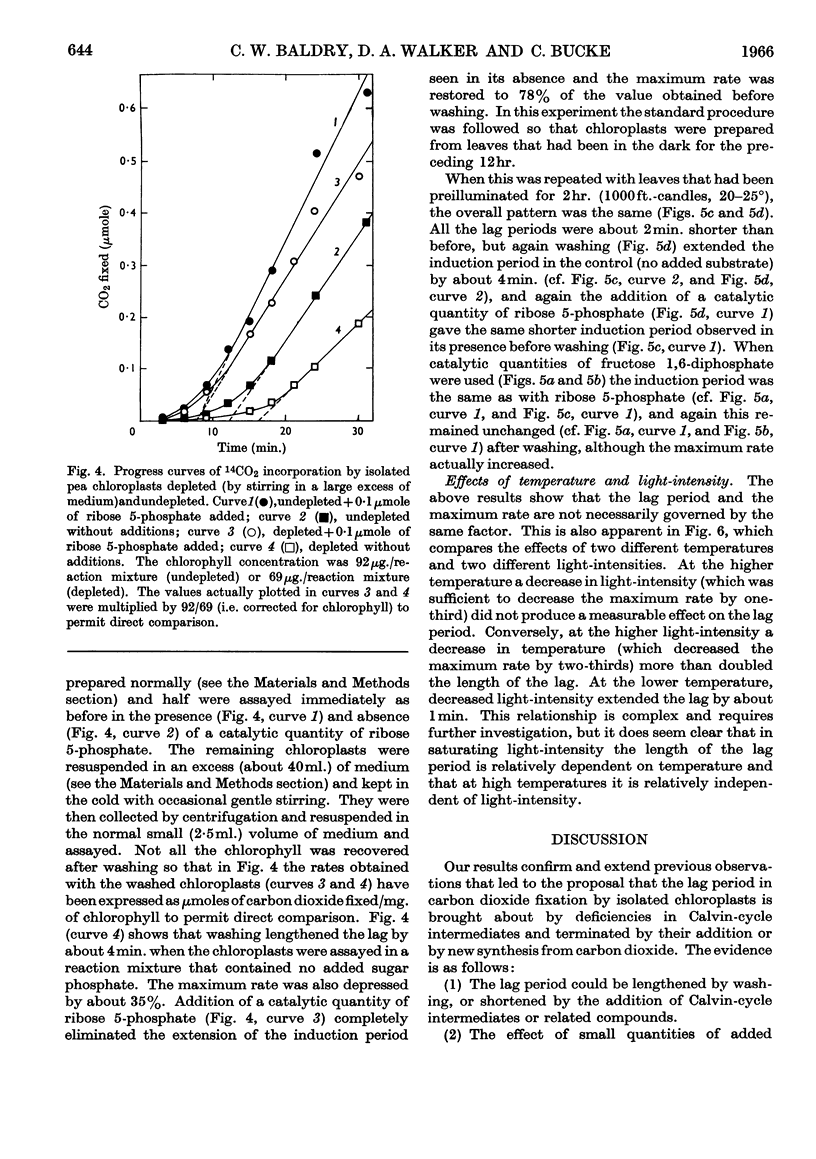
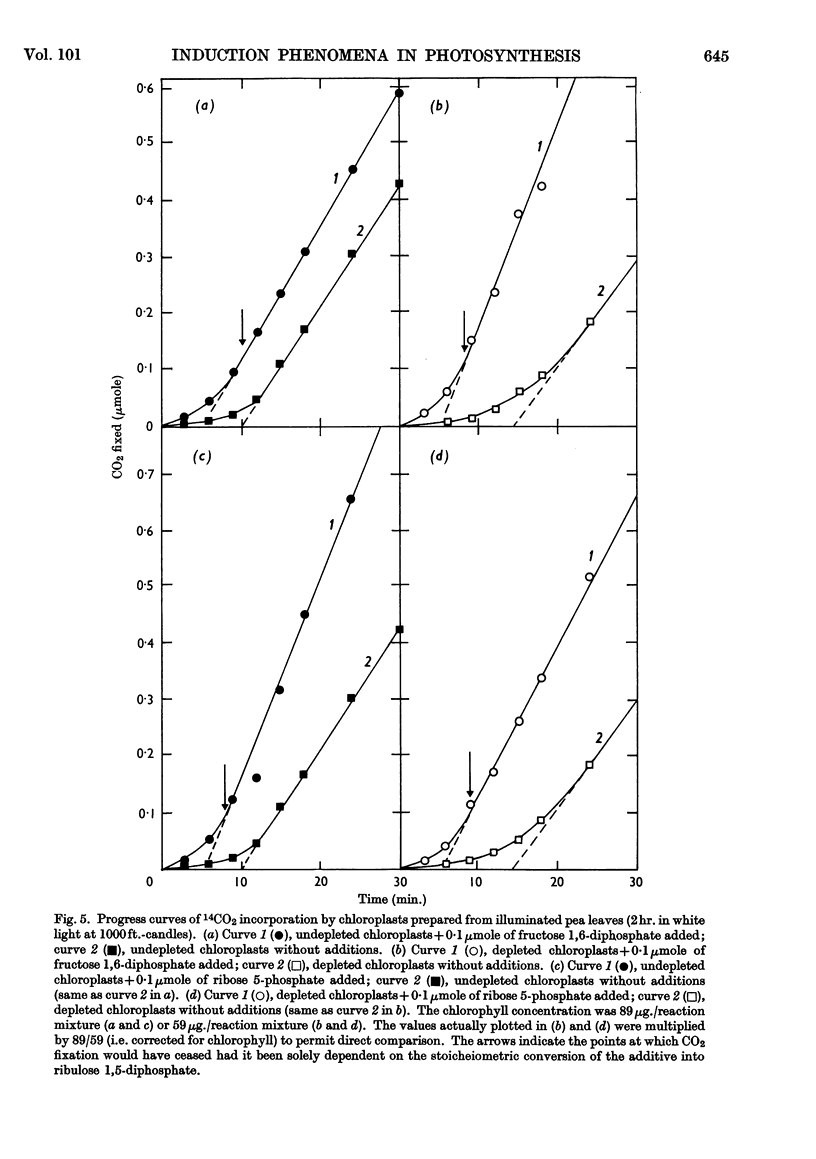
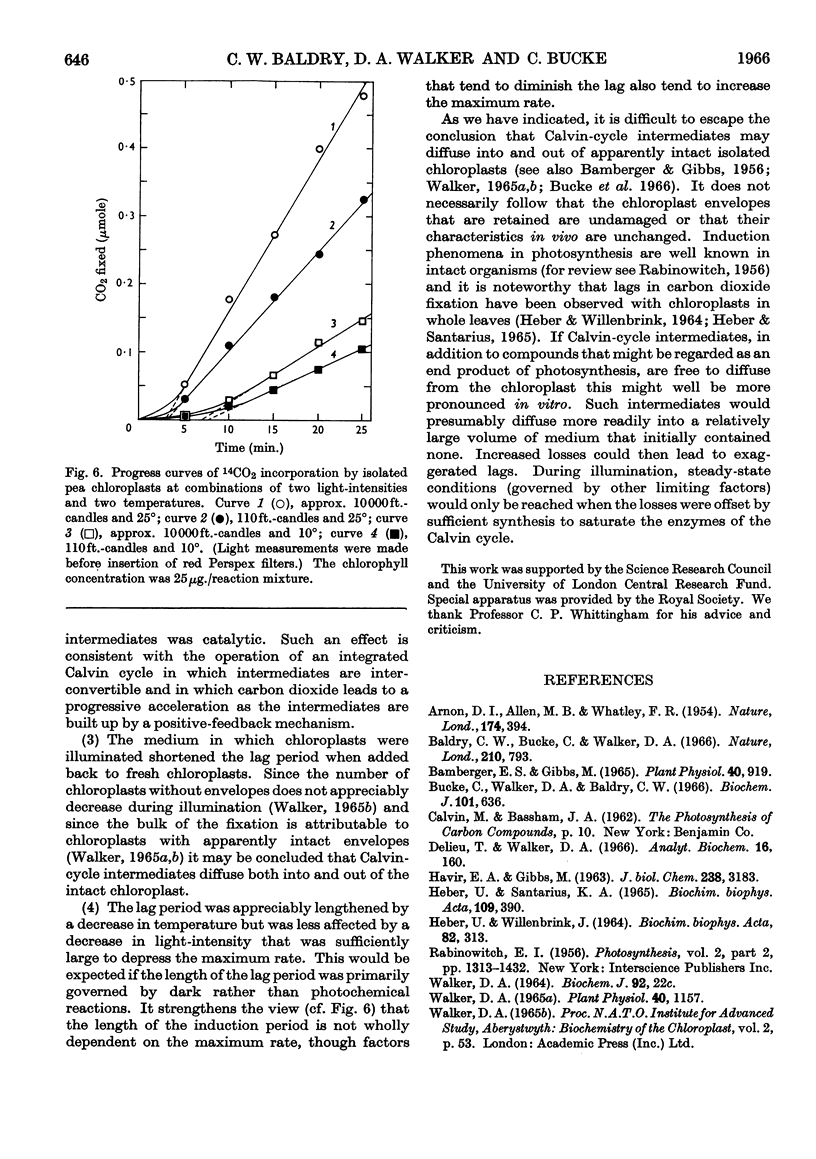
1. Induction periods in carbon dioxide fixation by isolated pea chloroplasts were shortened by small quantities of Calvin-cycle intermediates. The additional fixation was larger than that which would have followed direct stoicheiometric conversion into ribulose 1,5-diphosphate. 2. When chloroplasts were illuminated in the absence of added substrates (other than carbon dioxide) soluble products were formed in the medium that stimulated fixation by fresh chloroplasts. 3. The induction periods were lengthened by washing the chloroplasts. Addition of catalytic quantities of Calvin-cycle intermediates then decreased the induction periods to their previous values. 4. The induction period was extended by a decrease in temperature but was largely unaffected by a decrease in light-intensity that was sufficient to decrease the maximum rate. 5. It is concluded that the lag periods are a consequence of the loss of Calvin-cycle intermediates, such as sugar phosphates, through the intact chloroplast envelope and that these losses can be made good by new synthesis from carbon dioxide in the reactions of the Calvin cycle.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., ALLEN M. B., WHATLEY F. R. Photosynthesis by isolated chloroplasts. Nature. 1954 Aug 28;174(4426):394–396. doi: 10.1038/174394a0. [DOI] [PubMed] [Google Scholar]
- Bamberger E. S., Gibbs M. Effect of Phosphorylated Compounds and Inhibitors on CO(2) Fixation by Intact Spinach Chloroplasts. Plant Physiol. 1965 Sep;40(5):919–926. doi: 10.1104/pp.40.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucke C., Walker D. A., Baldry C. W. Some effects of sugars and sugar phosphates on carbon dioxide fixation by isolated chloroplasts. Biochem J. 1966 Dec;101(3):636–641. doi: 10.1042/bj1010636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delieu T., Walker D. A. An illuminated constant-temperature water bath for the study of photochemical reactions in biological systems. Anal Biochem. 1966 Jul;16(1):160–166. doi: 10.1016/0003-2697(66)90092-3. [DOI] [PubMed] [Google Scholar]
- HAVIR E. A., GIBBS M. STUDIES ON THE REDUCTIVE PENTOSE PHOSPHATE CYCLE IN INTACT AND RECONSTITUTED CHLOROPLAST SYSTEMS. J Biol Chem. 1963 Oct;238:3183–3187. [PubMed] [Google Scholar]
- HEBER U., WILLENBRINK J. SITES OF SYNTHESIS AND TRANSPORT OF PHOTOSYNTHETIC PRODUCTS WITHIN THE LEAF CELL. Biochim Biophys Acta. 1964 Feb 10;82:313–324. doi: 10.1016/0304-4165(64)90302-2. [DOI] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Walker D. A. Correlation between Photosynthetic Activity and Membrane Integrity in Isolated Pea Chloroplasts. Plant Physiol. 1965 Nov;40(6):1157–1161. doi: 10.1104/pp.40.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]


