Abstract
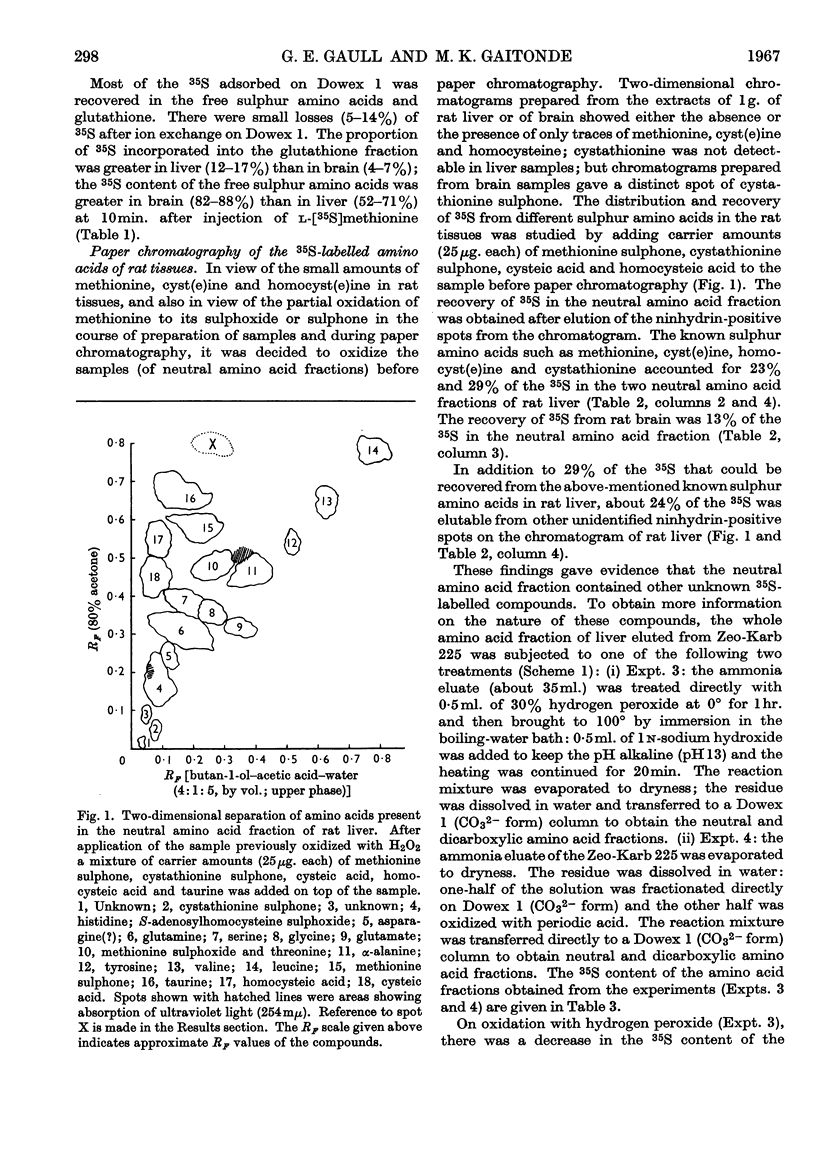
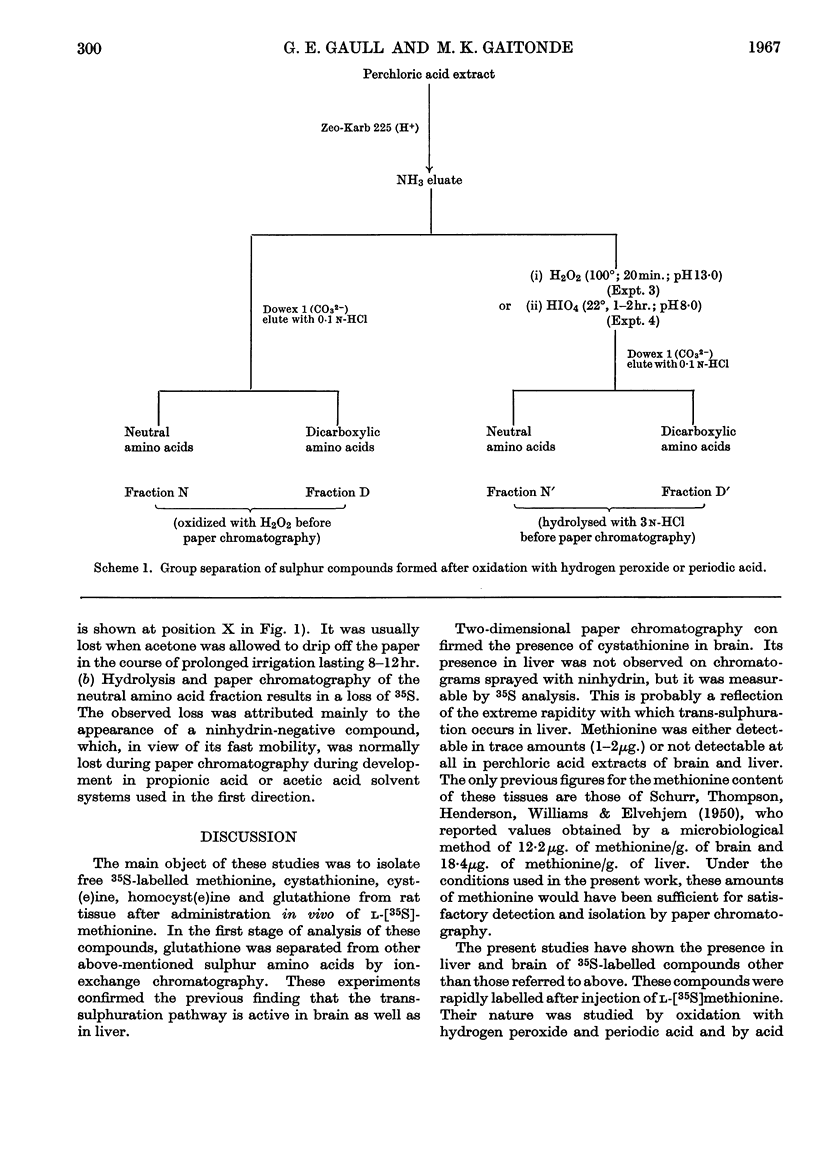
1. In a study of the metabolism of l-[35S]methionine in vivo, the labelled sulphur compounds of rat liver and brain were separated first by ion-exchange chromatography into two fractions containing (i) free sulphur amino acids such as methionine, cystathionine, cyst(e)ine and homocyst(e)ine and (ii) glutathione. 2. Two-dimensional paper chromatography with butan-1-ol–acetic acid or propionic acid–water in the first direction and 80% acetone or acetone–ethyl methyl ketone–water in the second direction was found superior to other solvent systems for separating the sulphur amino acids. 3. At 10min. after injection of [35S]methionine only a small part of the 35S was found combined in free methionine or other free sulphur amino acids. 4. Evidence was obtained of the presence of adenosyl[35S]methionine and adenosyl[35S]homocysteine in perchloric acid extracts of rat liver and brain. 5. The trans-sulphuration pathway was active in brain as well as in liver.
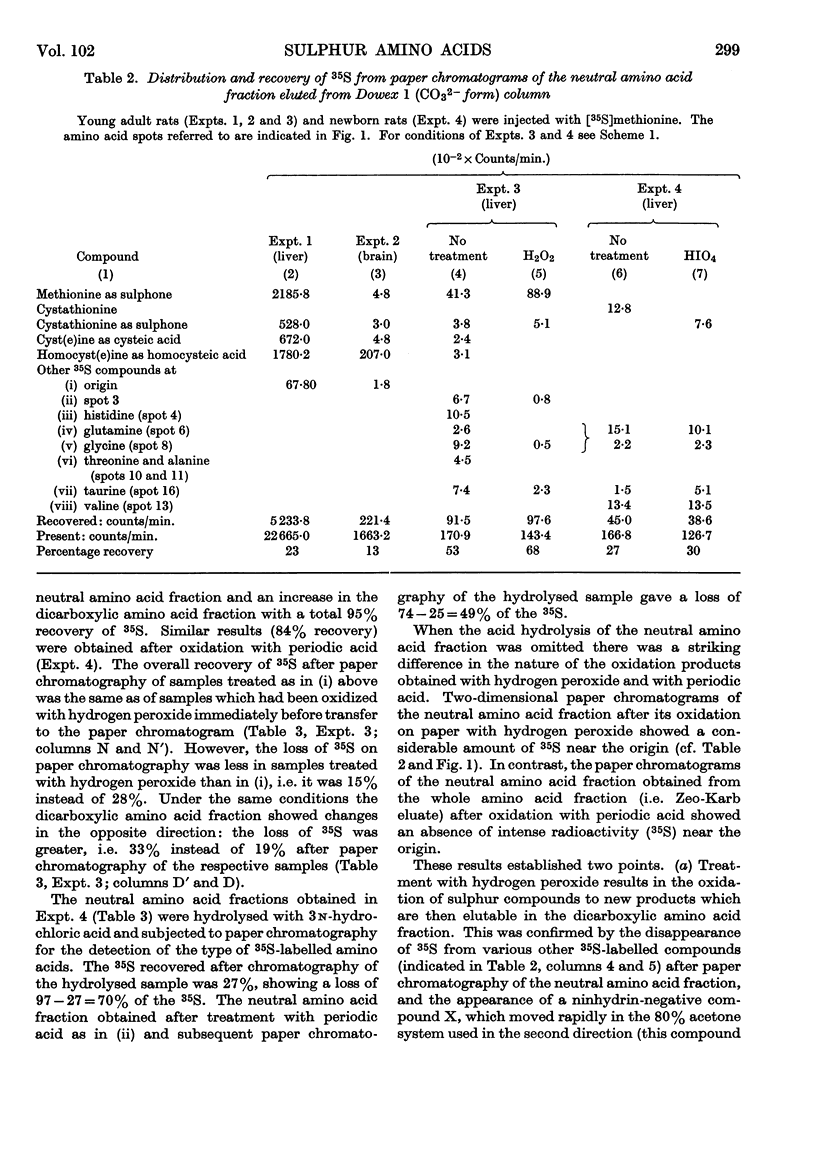
Full text
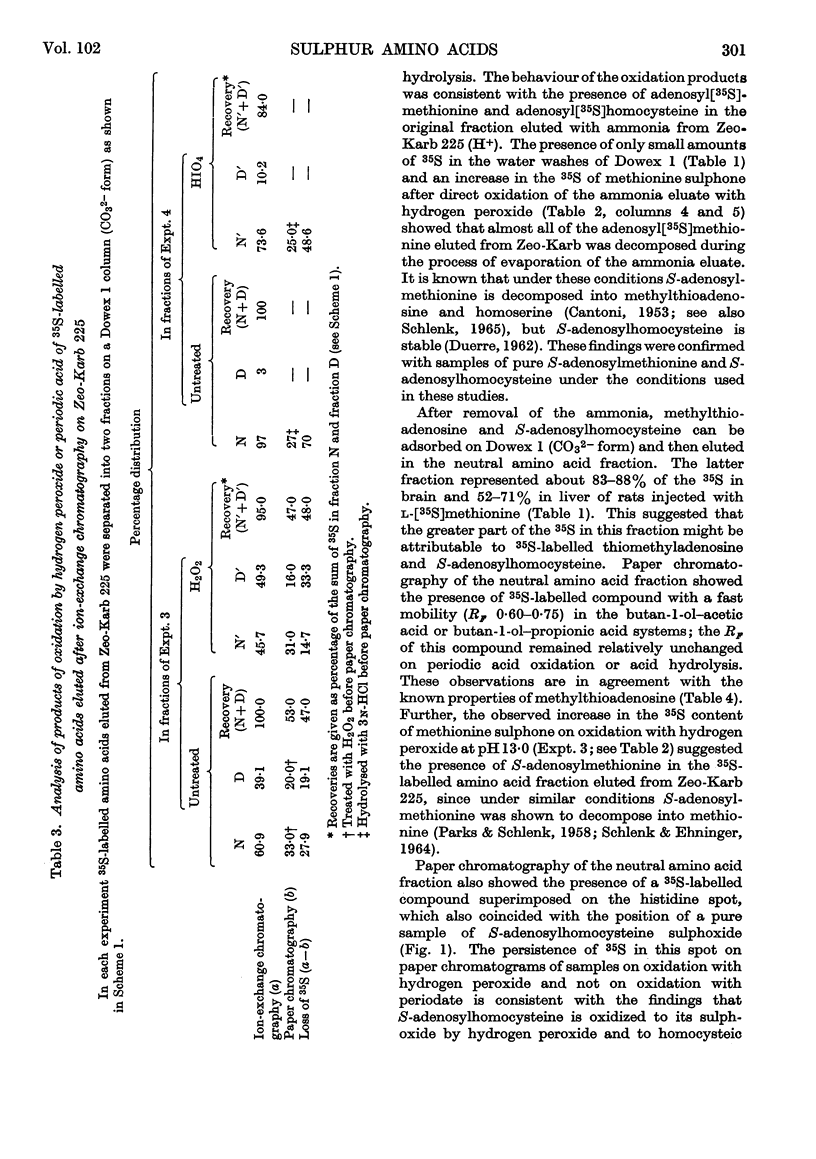
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Hudson R. F., Fox M. The reaction of oxidizing agents with wool. 1. The division of cystine into two fractions of widely differing reactivities. Biochem J. 1950 Jan;46(1):27–32. doi: 10.1042/bj0460027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSON N. A., DENT C. E., FIELD C. M., GAULL G. E. HOMOCYSTINURIA: CLINICAL AND PATHOLOGICAL REVIEW OF TEN CASES. J Pediatr. 1965 Mar;66:565–583. doi: 10.1016/s0022-3476(65)80121-4. [DOI] [PubMed] [Google Scholar]
- CLAMP J. R., HOUGH L. THE PERIODATE OXIDATION OF AMINO ACIDS WITH REFERENCE TO STUDIES ON GLYCOPROTEINS. Biochem J. 1965 Jan;94:17–24. doi: 10.1042/bj0940017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent C. E. A study of the behaviour of some sixty amino-acids and other ninhydrin-reacting substances on phenol-;collidine' filter-paper chromatograms, with notes as to the occurrence of some of them in biological fluids. Biochem J. 1948;43(2):169–180. [PMC free article] [PubMed] [Google Scholar]
- Dunstone J. R., Morgan W. T. Further observations on the glycoproteins in human ovarian cyst fluids. Biochim Biophys Acta. 1965 Nov 1;101(3):300–314. doi: 10.1016/0926-6534(65)90009-6. [DOI] [PubMed] [Google Scholar]
- GAITONDE M. K., DAHL D. R., ELLIOTT K. A. ENTRY OF GLUCOSE CARBON INTO AMINO ACIDS OF RAT BRAIN AND LIVER IN VIVO AFTER INJECTION OF UNIFORMLY 14-C-LABELLED GLUCOSE. Biochem J. 1965 Feb;94:345–352. doi: 10.1042/bj0940345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAITONDE M. K., GORDON M. W. A microchemical method for the detection and determination of shikimic acid. J Biol Chem. 1958 Feb;230(2):1043–1050. [PubMed] [Google Scholar]
- GAITONDE M. K. RATE OF UTILIZATION OF GLUCOSE AND 'COMPARTMENTATION' OF ALPHA-OXOGLUTARATE AND GLUTAMATE IN RAT BRAIN. Biochem J. 1965 Jun;95:803–810. doi: 10.1042/bj0950803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAITONDE M. K., RICHTER D. The metabolic activity of the proteins of the brain. Proc R Soc Lond B Biol Sci. 1956 Mar 27;144(918):83–99. doi: 10.1098/rspb.1956.0019. [DOI] [PubMed] [Google Scholar]
- GAITONDE M. K., RICHTER D. The uptake of 35S into tissues after injection of (35S) methionine. Biochem J. 1955 Apr;59(4):690–696. doi: 10.1042/bj0590690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAITONDE M. K. The composition of brain lipoprotein fractions obtained by different procedures. J Neurochem. 1961 Dec;8:234–242. doi: 10.1111/j.1471-4159.1961.tb13548.x. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K. The rate of incorporation of [S]methionine and [S]cystine into proteolipids and proteins of rat brain. Biochem J. 1961 Aug;80(2):277–284. doi: 10.1042/bj0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaull G., Gaitonde M. K. Homocystinuria: an observation on the inheritance of cystathionine synthase deficiency. J Med Genet. 1966 Sep;3(3):194–197. doi: 10.1136/jmg.3.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. Some new solvent systems for the paper chromatography of nucleic acid degradation products. Biochim Biophys Acta. 1955 Dec;18(4):575–576. doi: 10.1016/0006-3002(55)90157-8. [DOI] [PubMed] [Google Scholar]
- MUDD S. H., FINKELSTEIN J. D., IRREVERRE F., LASTER L. HOMOCYSTINURIA: AN ENZYMATIC DEFECT. Science. 1964 Mar 27;143(3613):1443–1445. doi: 10.1126/science.143.3613.1443. [DOI] [PubMed] [Google Scholar]
- PALLADIN A. V., POLIAKOVA N. M., SILICH T. P. Sravnitel'noe izuchenie belkov mozga i nerva. Fiziol Zh SSSR Im I M Sechenova. 1957 Jul;43(7):611–618. [PubMed] [Google Scholar]
- PARKS L. W., SCHLENK F. The stability and hydrolysis of S-adenosylmethionine; isolation of S-ribosylmethionine. J Biol Chem. 1958 Jan;230(1):295–305. [PubMed] [Google Scholar]
- SCHLENK F., DEPALMA R. E. The formation of S-adenosylmethionine in yeast. J Biol Chem. 1957 Dec;229(2):1037–1050. [PubMed] [Google Scholar]
- SCHRAM E., MOORE S., BIGWOOD E. J. Chromatographic determination of cystine as cysteic acid. Biochem J. 1954 May;57(1):33–37. doi: 10.1042/bj0570033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R. L., SCHLENK F. Determination of adenine thiomethylriboside and 5-thiomethylribose, and their differentiation from methionine. Arch Biochem Biophys. 1952 Jul;38:159–165. doi: 10.1016/0003-9861(52)90019-2. [DOI] [PubMed] [Google Scholar]
- STEVENS A., SAKAMI W. Biosynthesis of methionine in liver. J Biol Chem. 1959 Aug;234(8):2063–2072. [PubMed] [Google Scholar]
- Sanger F. Fractionation of oxidized insulin. Biochem J. 1949;44(1):126–128. doi: 10.1042/bj0440126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALLAN H. H., MOORE S., STEIN W. H. L-cystathionine in human brain. J Biol Chem. 1958 Feb;230(2):707–716. [PubMed] [Google Scholar]
- VRBA R., GAITONDE M. K., RICHTER D. The conversion of glucose carbon into protein in the brain and other organs of the rat. J Neurochem. 1962 Sep-Oct;9:465–475. doi: 10.1111/j.1471-4159.1962.tb04199.x. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]


