Abstract
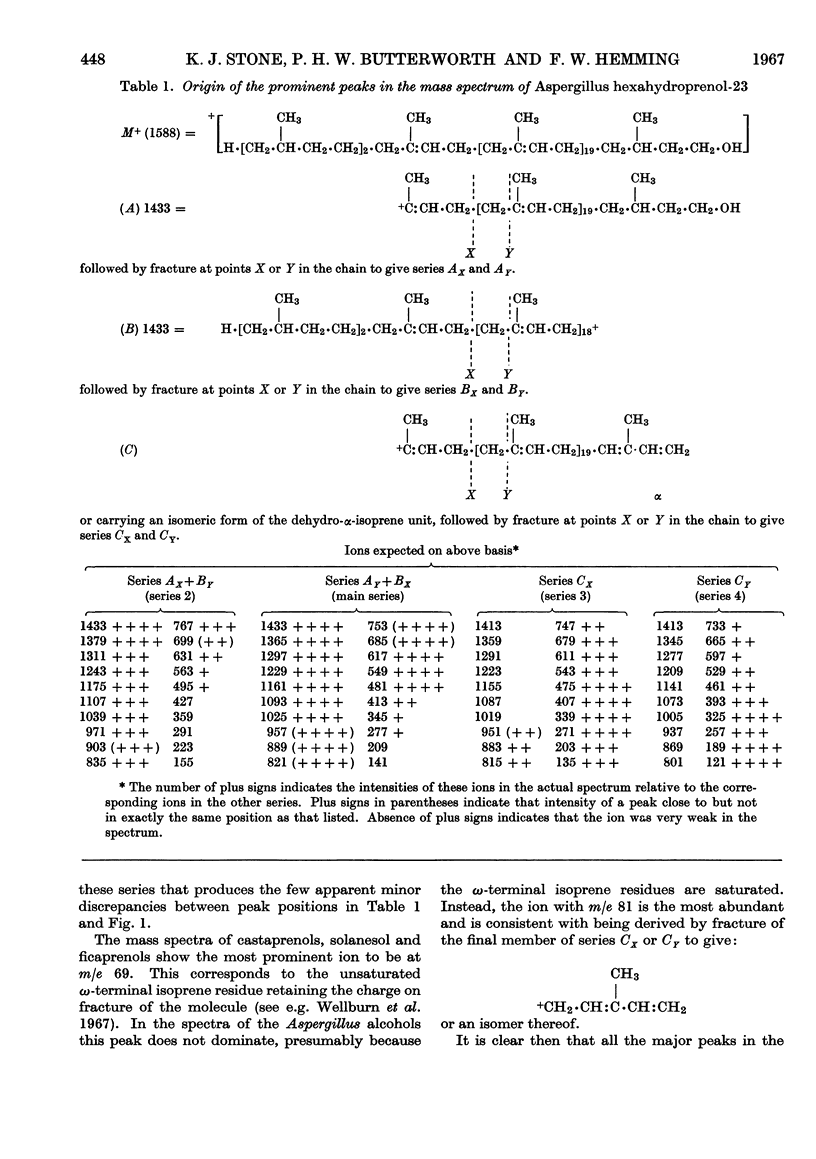
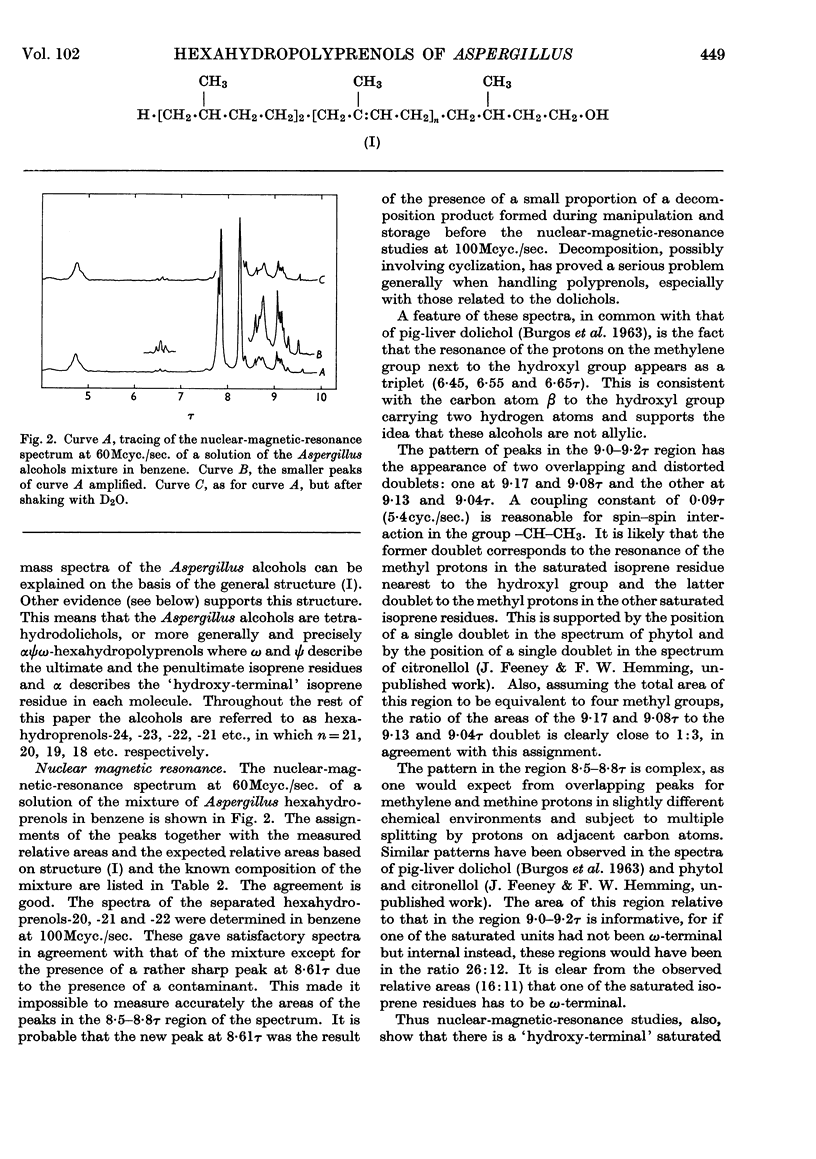
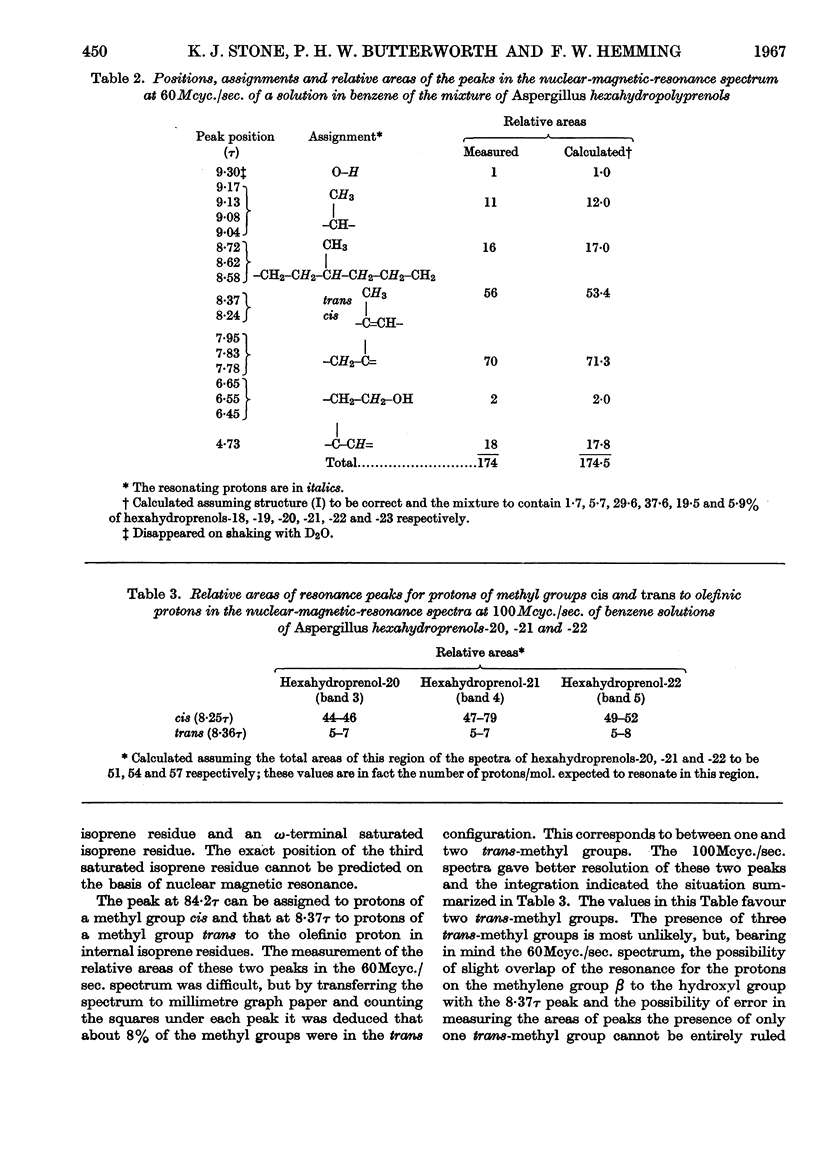
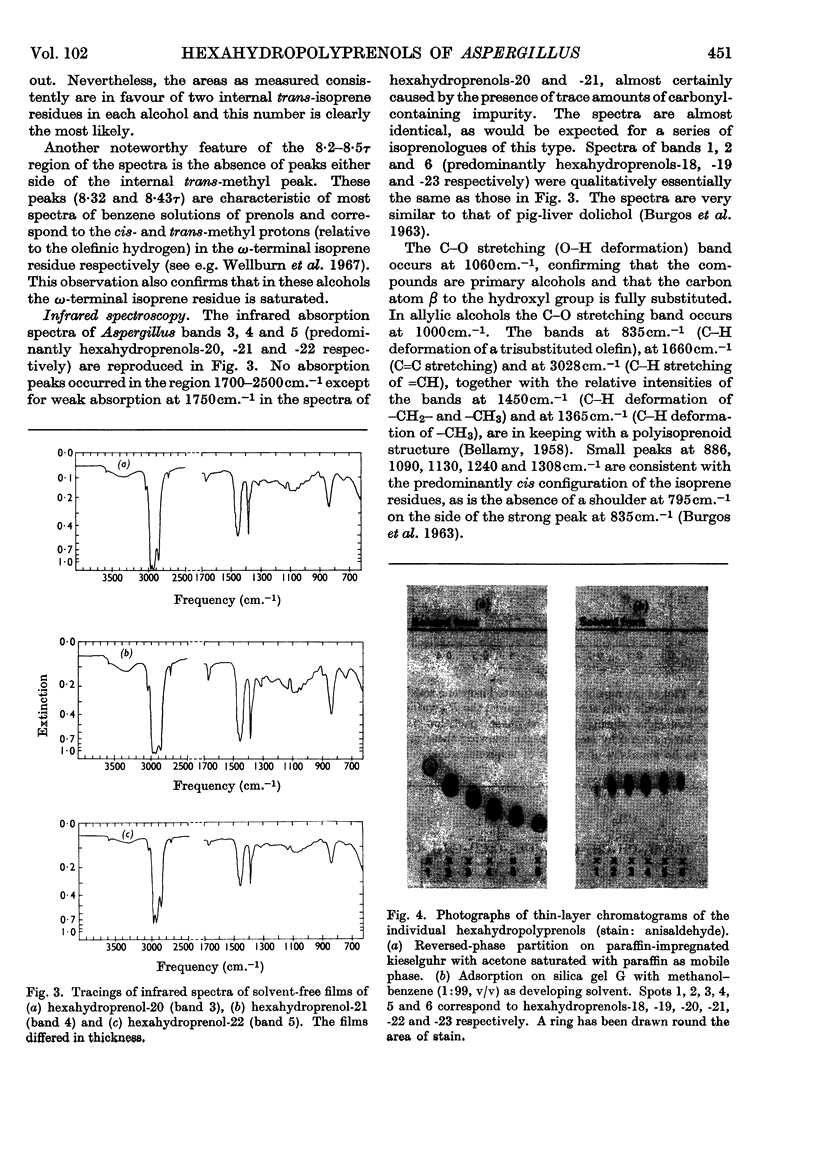
The isolation and properties of a group of alcohols from the mycelium of Aspergillus fumigatus Fresenius are described. Mass-, nuclear-magnetic-resonance- and infrared-spectrometric studies coupled with evidence from ozonolytic degradation and chromatography show the mixture to contain hexahydroprenols-18, -19, -20, -21, -22, -23 and -24. Each contains a saturated `hydroxy-terminal' isoprene residue, a saturated ω-terminal isoprene residue and a saturated ζ-isoprene residue (adjacent to the ω-residue). The presence of only two trans-isoprene residues is also a feature of the series of alcohols, but the precise position of these in each molecule is not known.
Full text
PDF


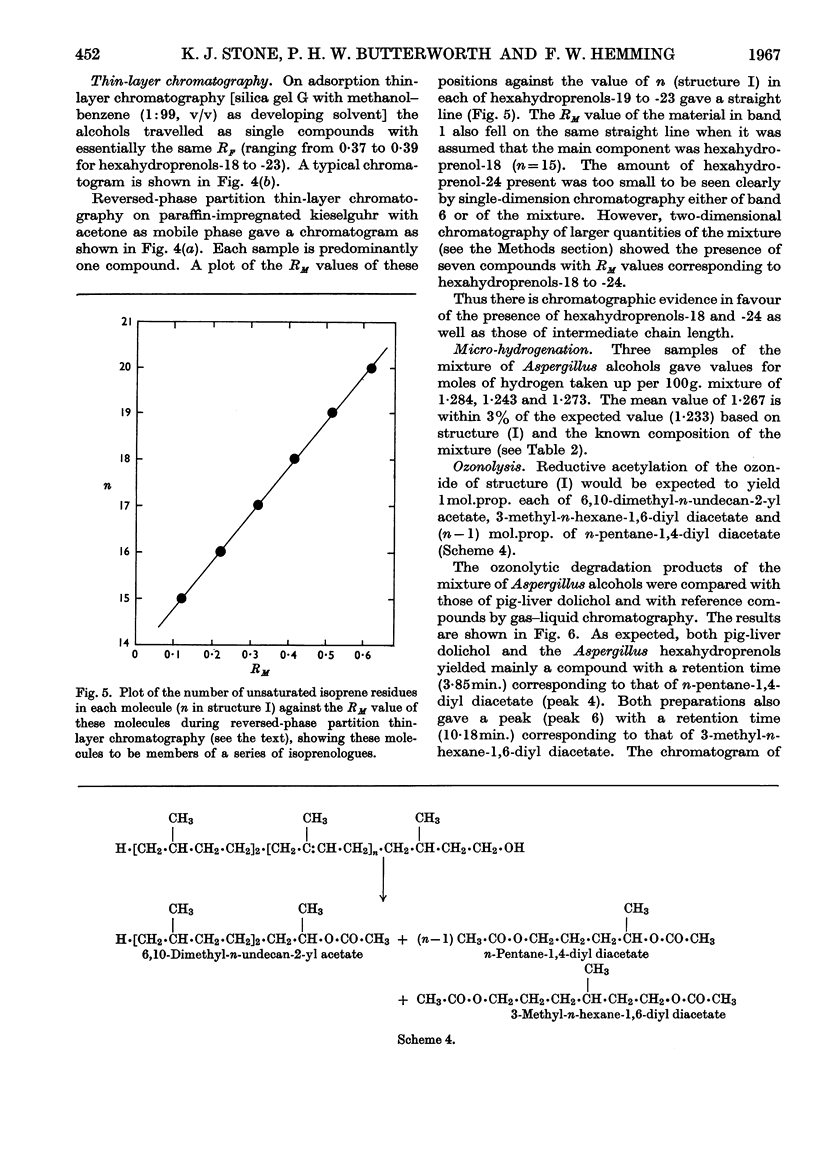
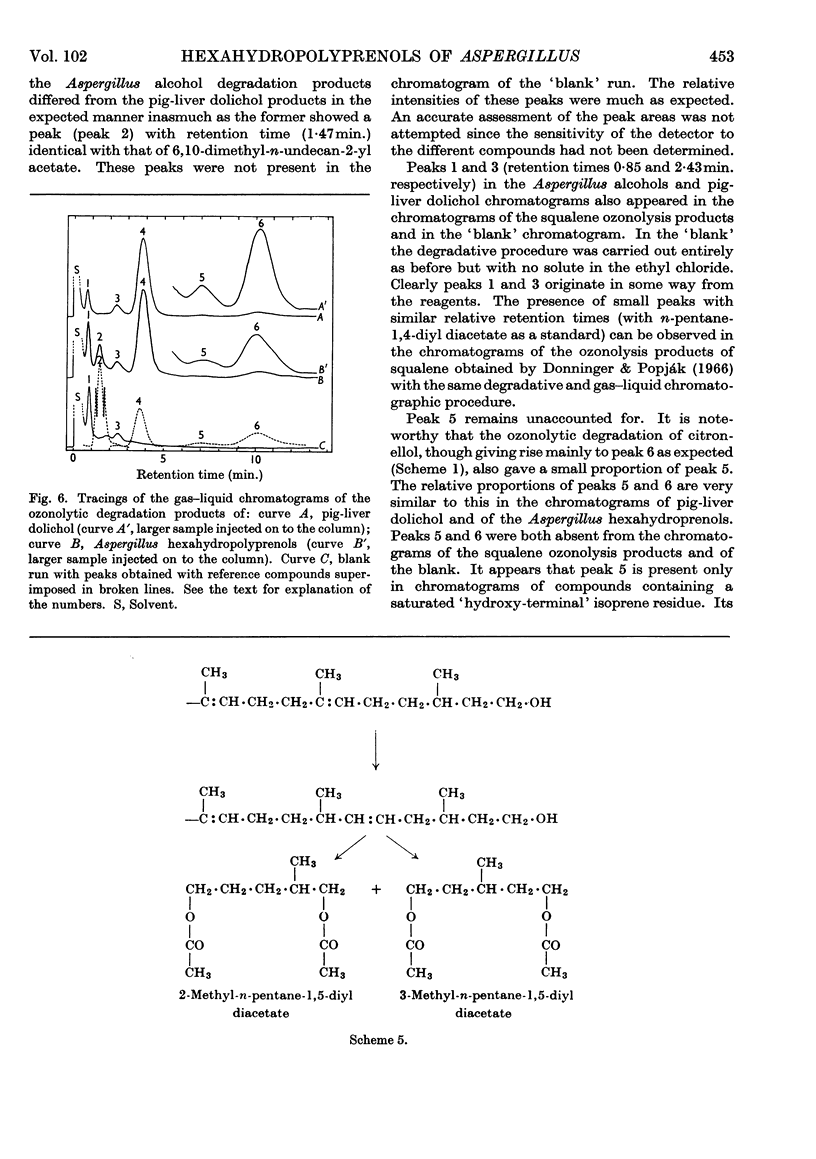


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anslow W. K., Raistrick H. Studies in the biochemistry of micro-organisms: Fumigatin (3-hydroxy-4-methoxy-2:5-toluquinone), and spinulosin (3:6-dihydroxy-4-methoxy-2:5-toluquinone), metabolic products respectively of Aspergillus fumigatus Fresenius and Penicillium spinulosum Thom. Biochem J. 1938 Apr;32(4):687–696. doi: 10.1042/bj0320687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGOS J., HEMMING F. W., PENNOCK J. F., MORTON R. A. DOLICHOL: A NATURALLY-OCCURRING C100 ISOPRENOID ALCOHOL. Biochem J. 1963 Sep;88:470–482. [PMC free article] [PubMed] [Google Scholar]
- Donninger C., Popják G. Studies on the biosynthesis of cholesterol. 18. The stereospecificity of mevaldate reductase and the biosynthesis of asymmetrically labelled farnesyl pyrophosphate. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):465–491. doi: 10.1098/rspb.1966.0003. [DOI] [PubMed] [Google Scholar]
- Stone K. J., Wellburn A. R., Hemming F. W., Pennock J. F. The characterization of ficaprenol-10, -11 and 12 from the leaves of Ficus elastica (decorative rubber plant). Biochem J. 1967 Jan;102(1):325–330. doi: 10.1042/bj1020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Kodicek E. The structure of bactoprenol, a lipid formed by lactobacilli from mevalonic acid. Biochem J. 1966 Apr;99(1):123–127. doi: 10.1042/bj0990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn A. R., Stevenson J., Hemming F. W., Morton R. A. The characterization and properties of castaprenol-11, -12 and -13 from the leaves of Aesculus hippocastanum (horse chestnut). Biochem J. 1967 Jan;102(1):313–324. doi: 10.1042/bj1020313. [DOI] [PMC free article] [PubMed] [Google Scholar]