Abstract
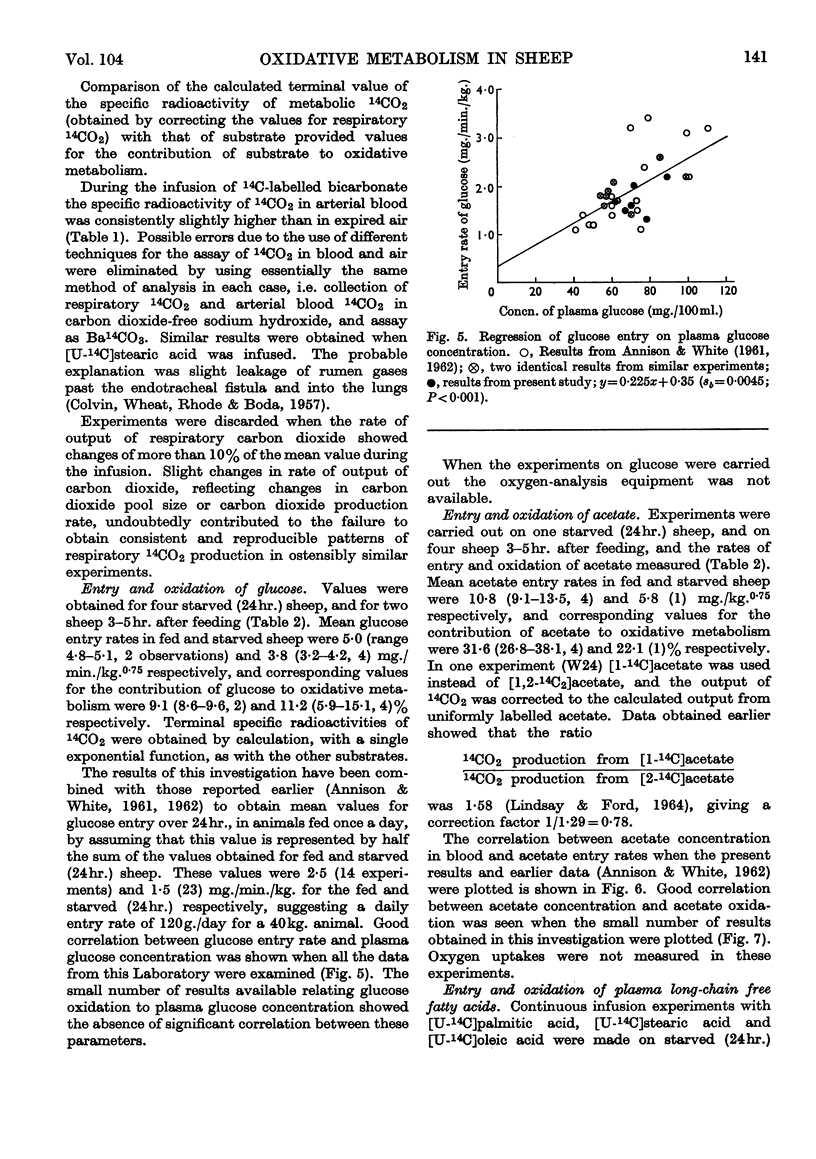
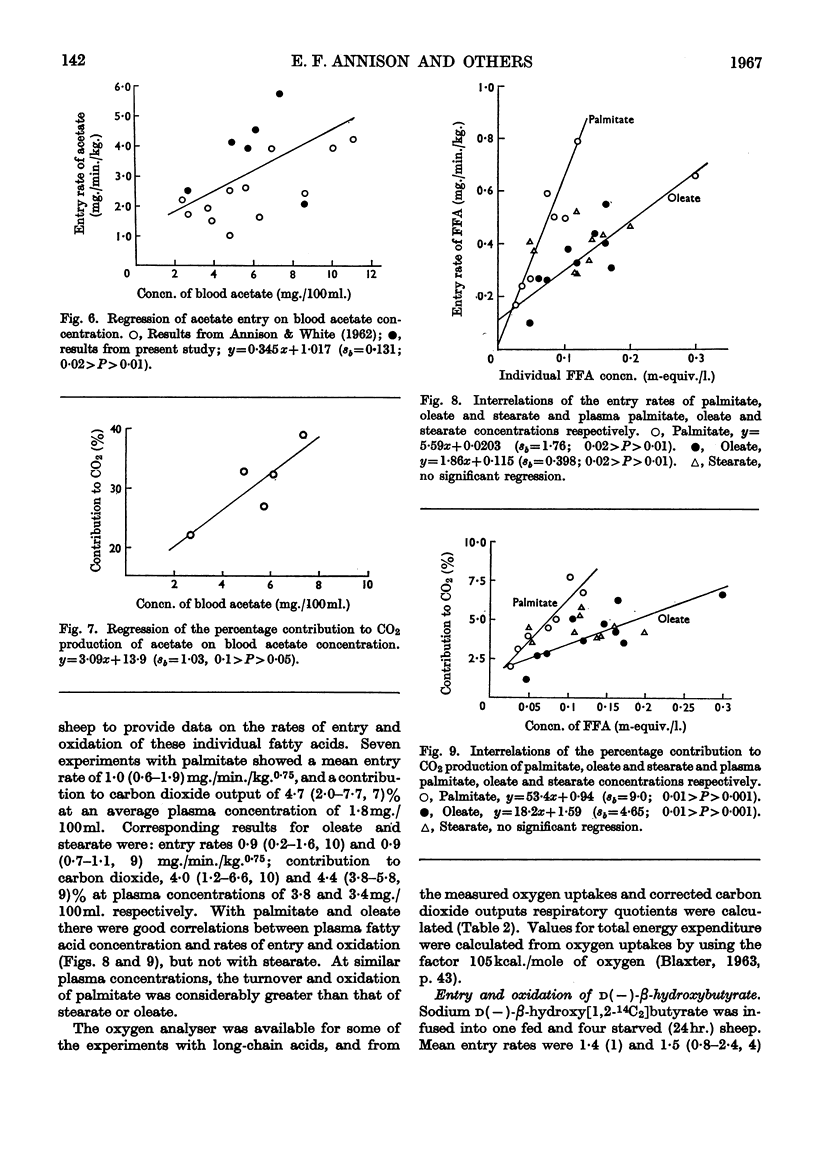
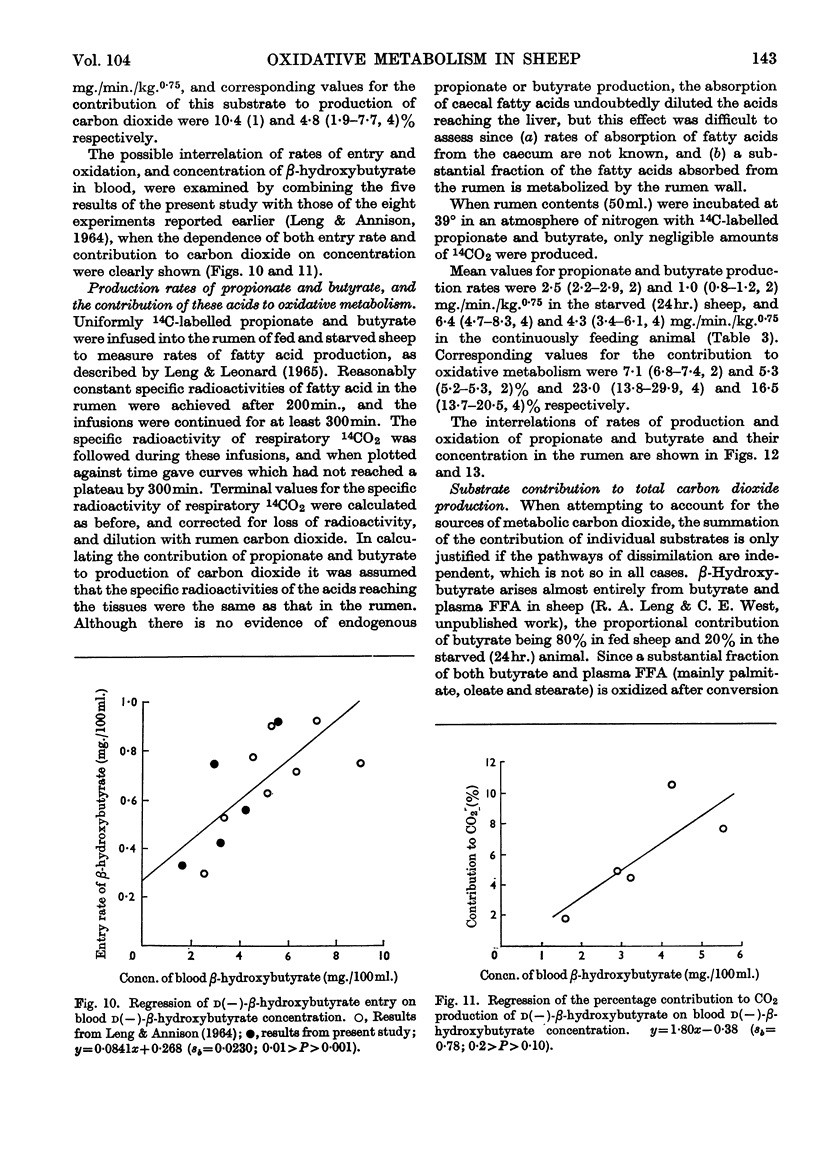
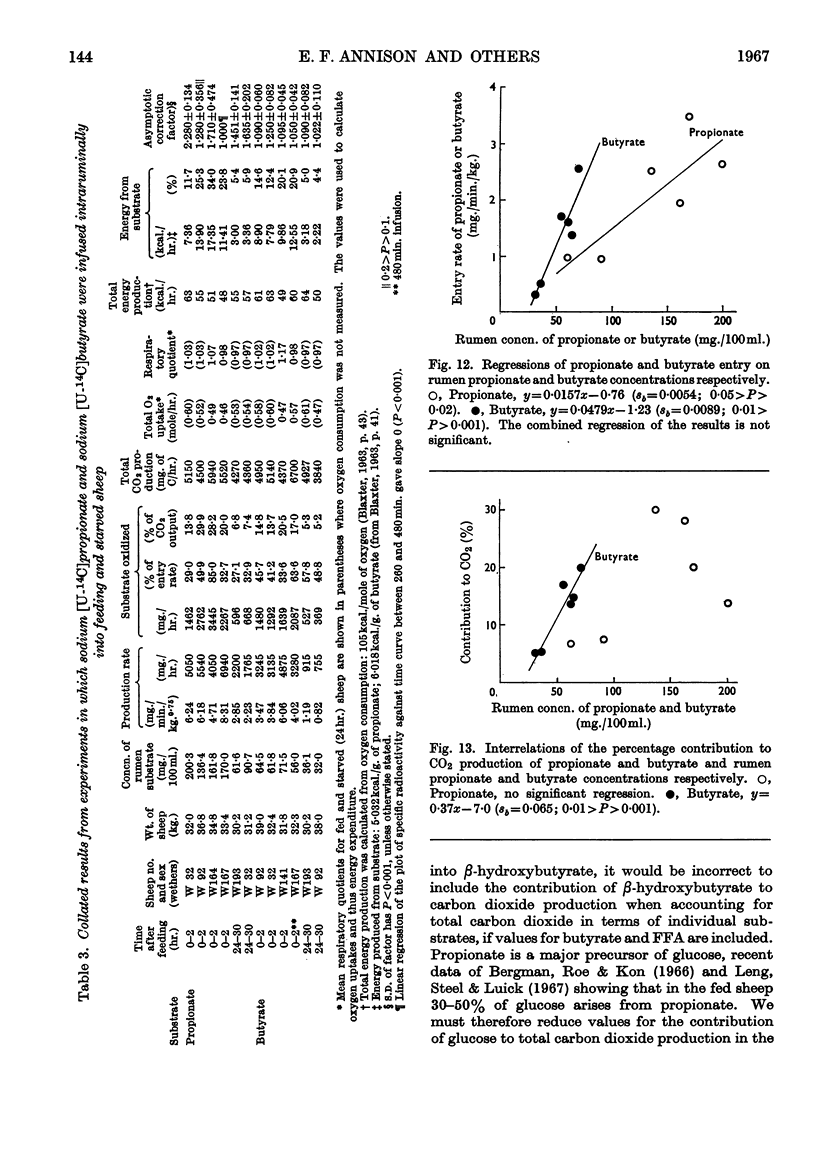
1. Rates of entry and oxidation of a range of metabolites have been measured in tracheostomized sheep (diet, 800g. of lucerne chaff and 100g. of maize/day) by combining isotope-dilution techniques with the continuous measurement of total respiratory gas exchange, and 14CO2 production during the intravenous or intraruminal infusion of 14C-labelled substrates. 2. Mean entry rates in fed and starved (24hr.) sheep respectively, expressed as mg./min./kg. body wt.0·75, were: glucose, 5·0 (range 4·8–5·1, 2 observations) and 3·8 (3·2–4·2, 4); acetate, 10·8 (9·1–13·5, 4) and 5·8 (1); d(−)-β-hydroxybutyrate, 1·4 (1) and 1·5 (0·8–2·4, 4); palmitate, oleate and stearate (starved sheep only) 1·0 (0·6–1·9, 7), 0·9 (0·2–1·6, 10) and 0·9 (0·5–1·1, 11) respectively. 3. Production rates of propionate and butyrate in continuously feeding sheep were 6·4 (4·7–8·3, 4) and 4·3 (3·4–6·1, 4) mg./min./kg.0·75 respectively, and in starved (24hr.) sheep were 2·5 (2·2–2·9, 2) and 1·0 (0·8–1·2, 2) mg./min./kg.0·75 respectively. 4. Calculated terminal values for the specific radioactivity of respiratory 14CO2 during measurements of entry rates and production rates were used to calculate the contributions of individual substrates to overall oxidative metabolism. Mean values for fed and starved sheep respectively were: glucose, 9·1 (8·6–9·6, 2) and 11·2 (5·9–15·1, 4)%; acetate, 31·6 (26·8–38·1, 4) and 22·1 (1)%; d(−)-β-hydroxybutyrate, 10·4 (1) and 4·8 (1·9–7·7, 4)%; propionate, 23·0 (13·8–29·9, 4) and 7·1 (6·8–7·4, 2)%; butyrate, 16·5 (13·7–20·5, 4) and 5·3 (5·2–5·3, 2)%; palmitate, oleate and stearate (starved sheep only), 4·7 (2·0–7·7, 7), 4·0 (1·2–6·6, 10) and 4·4 (3·8–5·8, 9)% respectively. The sum of these values for individual substrates in fed and starved sheep, excluding that of β-hydroxybutyrate and after correction of the glucose value for the known interrelations of this substrate with propionate, accounted for 76% and 58% respectively of total production of carbon dioxide. 5. Calculations based on the proportion of substrate entry directly oxidized indicated that the substrates studied accounted for 63% (fed sheep) and 43% (starved sheep) of total energy expenditure measured by oxygen uptake. The contribution of β-hydroxybutyrate was excluded, and corrections were made for glucose–propionate interrelations, and for the different rates of oxidation of the methyl and carboxyl fragments of acetate. 6. The present results have been combined with those obtained earlier in this Laboratory to examine the relationships between rates of substrate entry and oxidation, and concentrations of substrate in blood. Rates of entry of acetate, glucose, d(−)-β-hydroxybutyrate, palmitate and oleate (but not stearate) were well correlated with concentration in blood, and substrate contribution to production of carbon dioxide showed a similar correlation to blood concentration, except with glucose. 7. It was concluded that the general technique is of potential value in providing valid quantitative parameters of animal metabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F., LINDSAY D. B. Acetate utilization in sheep. Biochem J. 1961 Apr;78:777–785. doi: 10.1042/bj0780777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F., LINDSAY D. B. The measurement of entry rates of propionate and of butyrate in sheep. Biochem J. 1962 Dec;85:474–479. doi: 10.1042/bj0850474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F., LINDSAY D. B., WHITE R. R. METABOLIC INTERRELATIONS OF GLUCOSE AND LACTATE IN SHEEP. Biochem J. 1963 Aug;88:243–248. doi: 10.1042/bj0880243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F., WHITE R. R. Further studies on the entry rates of acetate and glucose in sheep, with special reference to endogenous production of acetate. Biochem J. 1962 Sep;84:546–552. doi: 10.1042/bj0840546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANNISON E. F., WHITE R. R. Glucose utilization in sheep. Biochem J. 1961 Jul;80:162–169. doi: 10.1042/bj0800162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAXTER K. L., JOYCE J. P. THE ACCURACY AND EASE WITH WHICH MEASUREMENTS OF RESPIRATORY METABOLISM CAN BE MADE WITH TRACHEOSTOMIZED SHEEP. Br J Nutr. 1963;17:523–537. doi: 10.1079/bjn19630055. [DOI] [PubMed] [Google Scholar]
- Bergman E. N., Reid R. S., Murray M. G., Brockway J. M., Whitelaw F. G. Interconversions and production of volatile fatty acids in the sheep rumen. Biochem J. 1965 Oct;97(1):53–58. doi: 10.1042/bj0970053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman E. N., Roe W. E., Kon K. Quantitative aspects of propionate metabolism and gluconeogenesis in sheep. Am J Physiol. 1966 Sep;211(3):793–799. doi: 10.1152/ajplegacy.1966.211.3.793. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., DAVIES R. E., WOOD D. R. The metabolism of 14C-labelled bicarbonate in the cat. Biochem J. 1952 Jun;51(3):351–357. doi: 10.1042/bj0510351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL S. Turnover rate of unesterified fatty acids in human plasma. Acta Physiol Scand. 1957 Dec 7;41(2-3):158–167. doi: 10.1111/j.1748-1716.1957.tb01517.x. [DOI] [PubMed] [Google Scholar]
- Leng R. A., Annison E. F. The metabolism of D(--)-beta-hydroxybutyrate in sheep. Biochem J. 1964 Mar;90(3):464–469. doi: 10.1042/bj0900464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng R. A. Ketone body metabolism in normal and underfed pregnant sheep and in pregnancy toxaemia. Res Vet Sci. 1965 Oct;6(4):433–441. [PubMed] [Google Scholar]
- Leng R. A., Leonard G. J. Measurement of the rates of production of acetic, propionic and butyric acids in the rumen of sheep. Br J Nutr. 1965;19(4):469–484. doi: 10.1079/bjn19650043. [DOI] [PubMed] [Google Scholar]
- Leng R. A., Steel J. W., Luick J. R. Contribution of propionate to glucose synthesis in sheep. Biochem J. 1967 Jun;103(3):785–790. doi: 10.1042/bj1030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D. B., Brown R. E. Acetone metabolism in sheep. Biochem J. 1966 Sep;100(3):589–592. doi: 10.1042/bj1000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D. B., Ford E. J. Acetate utilization and the turnover of citric acid-cycle components in pregnant sheep. Biochem J. 1964 Jan;90(1):24–30. doi: 10.1042/bj0900024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R. The retention of metabolic radioactive carbonate. Biochem J. 1955 Jul;60(3):447–453. doi: 10.1042/bj0600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D. Fatty acid mobilization--mechanisms of regulation and metabolic consequences. Biochem Soc Symp. 1963;24:111–143. [PubMed] [Google Scholar]
- TOLBERT B. M., KIRK M., BAKER E. M. Continuous C14O2 and CO2 excretion studies in experimental animals. Am J Physiol. 1956 May;185(2):269–274. doi: 10.1152/ajplegacy.1956.185.2.269. [DOI] [PubMed] [Google Scholar]
- West C. E., Annison E. F. Metabolism of palmitate in sheep. Biochem J. 1964 Sep;92(3):573–578. doi: 10.1042/bj0920573. [DOI] [PMC free article] [PubMed] [Google Scholar]


