Abstract
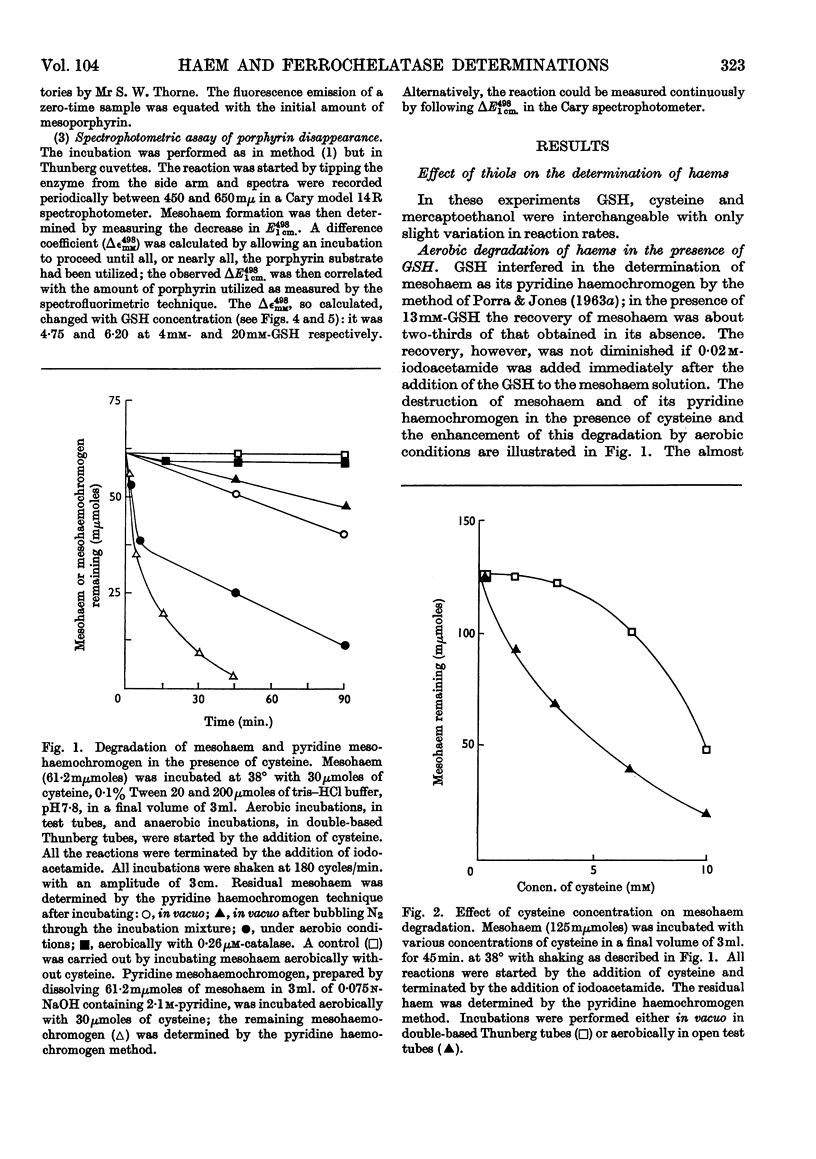
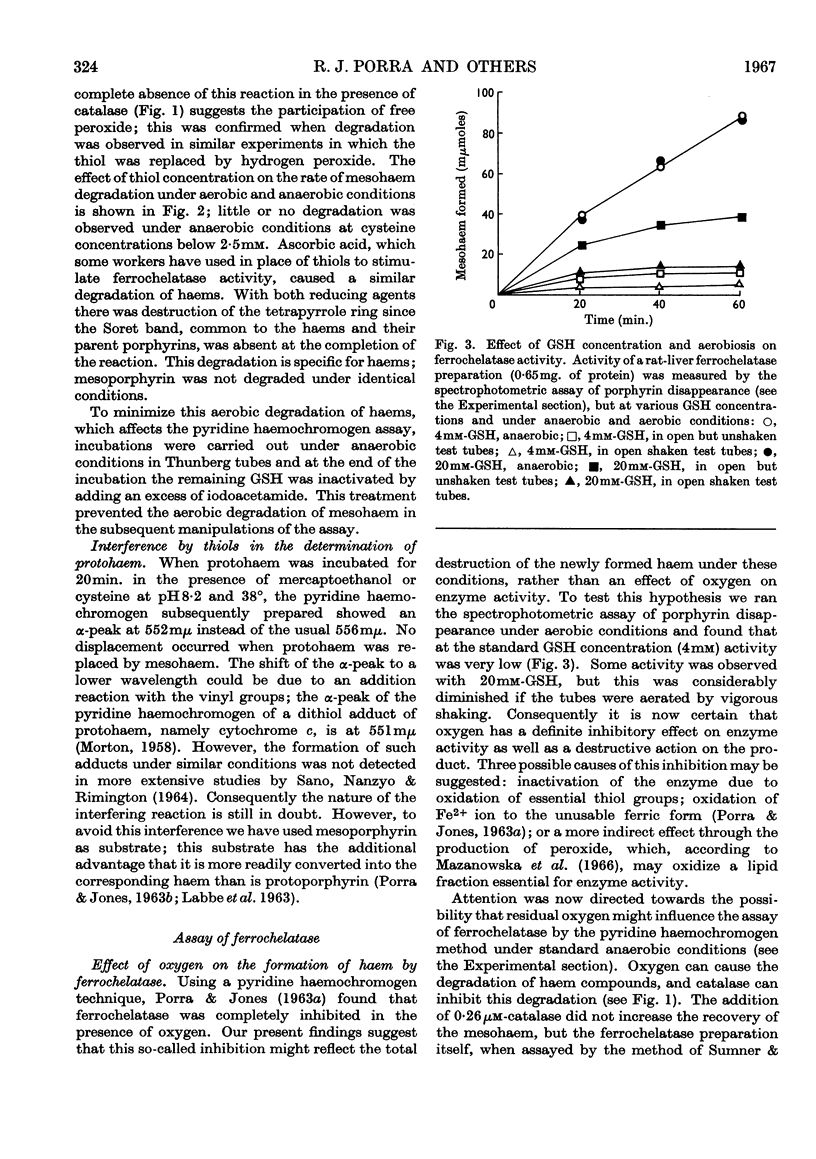
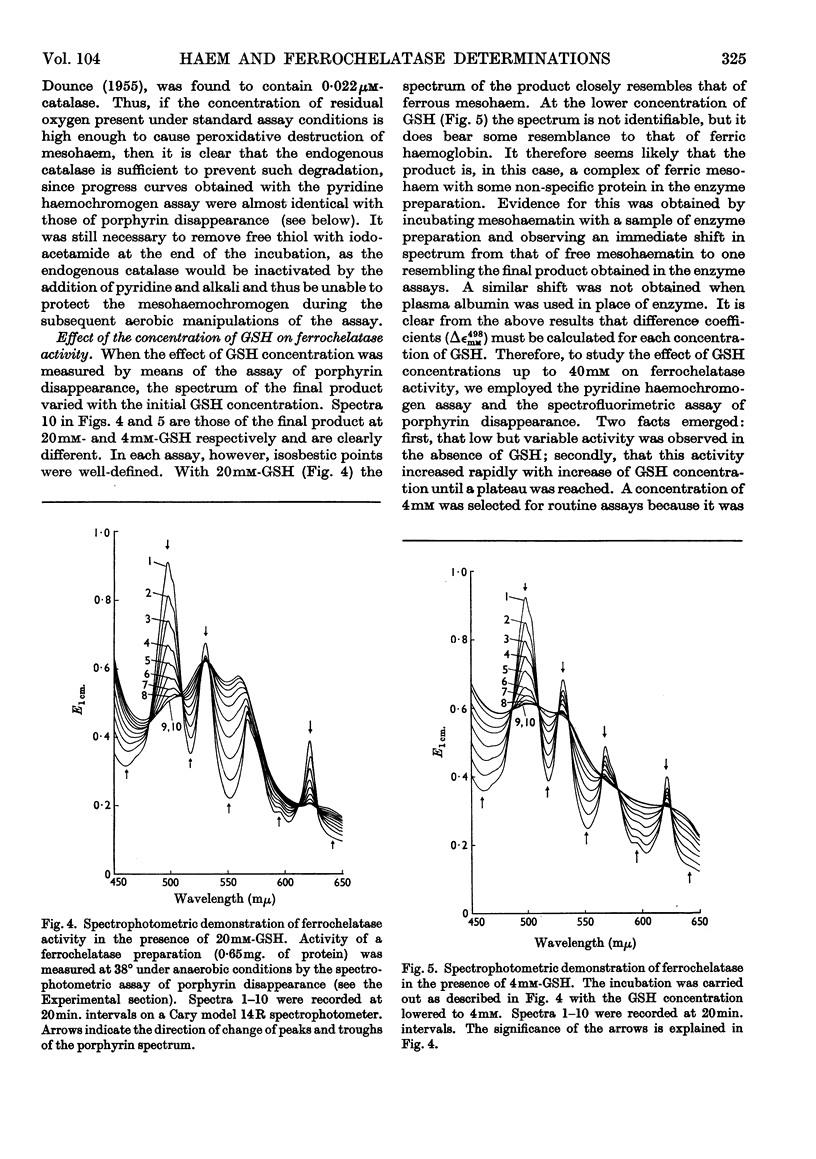
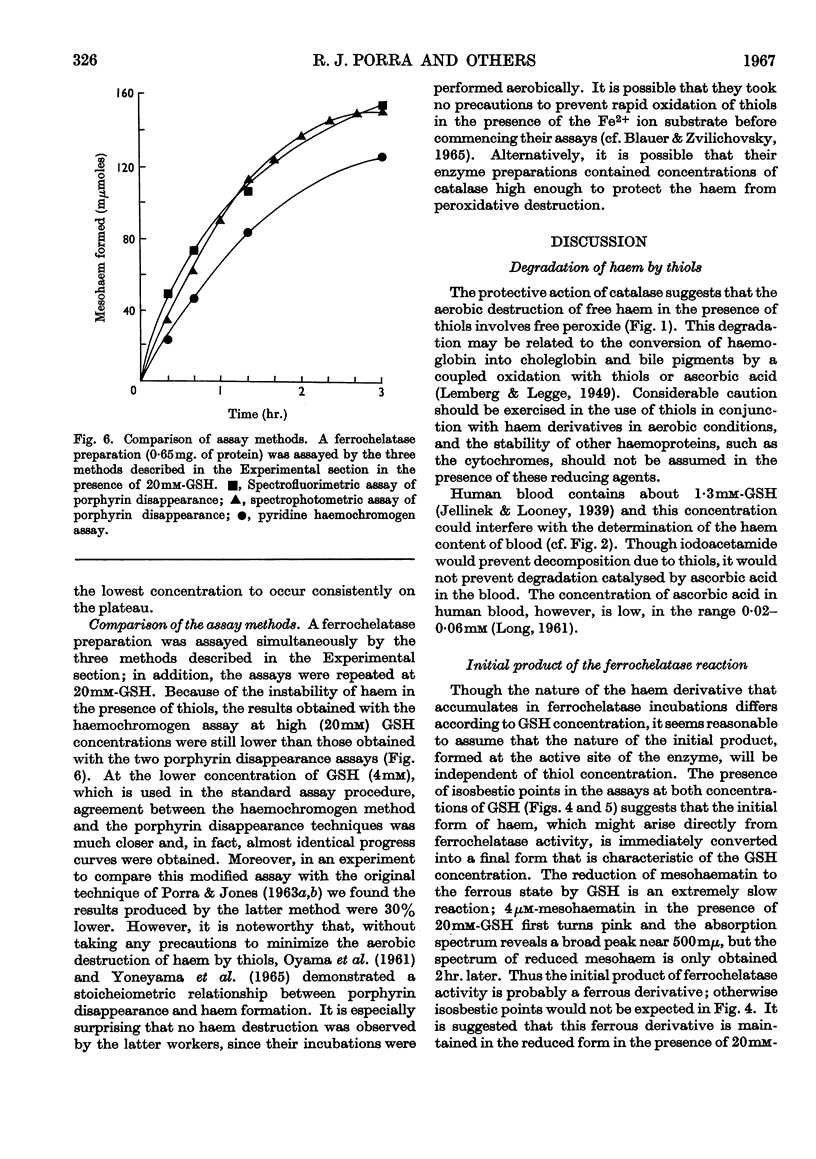
1. Haems are unstable under aerobic conditions in the presence of thiols, which are used to activate the ferrochelatase enzyme; catalase inhibits this degradation of haem. In addition, thiols interfere with the determination of protohaem as its pyridine haemochromogen derivative. 2. Three ferrochelatase assays are described that minimize interference by these two reactions. Two of these assays involve measurement of porphyrin utilization, one spectrophotometrically and the second spectrofluorimetrically. The third assay measures haem formation by a pyridine haemochromogen technique. Results obtained with these three methods were in close agreement at a GSH concentration of 4mm. 3. The stimulatory effect of GSH on ferrochelatase has been confirmed. The spectrum of the haem formed is dependent on GSH concentration; at high GSH concentrations (20mm) the haem is in the reduced state, but at low concentration (4mm) the spectrum of the product resembles that of an oxidized haemoprotein such as ferrihaemoglobin. 4. The inhibitory effect of oxygen on ferrochelatase activity has been confirmed by spectrophotometric assay of porphyrin disappearance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHENBRUCKER H., CARTWRIGHT G. E., GOLDBERG A., WINTROBE M. M. Studies on the biosynthesis of heme in vitro by avian erythrocytes. Blood. 1956 Sep;11(9):821–833. [PubMed] [Google Scholar]
- Blauer G., Zvilichovsky B. Marcromolecular complexes of ferriprotoporphyrin IX as catalysts for the oxidation of cysteine by molecular oxygen. Biochim Biophys Acta. 1965 Oct 25;110(1):215–217. doi: 10.1016/s0926-6593(65)80116-3. [DOI] [PubMed] [Google Scholar]
- JOHNSON A., JONES O. G. ENZYMIC FORMATION OF HAEMS AND OTHER METALLOPORPHYRINS. Biochim Biophys Acta. 1964 Oct 9;93:171–173. doi: 10.1016/0304-4165(64)90273-9. [DOI] [PubMed] [Google Scholar]
- KLEIN J. R., KRUEGER R. C., MELNICK I. Formation of heme by broken-cell preparations of duck erythrocytes. Arch Biochem Biophys. 1956 Oct;64(2):302–310. doi: 10.1016/0003-9861(56)90273-9. [DOI] [PubMed] [Google Scholar]
- LABBE R. F. An enzyme which catalyzes the insertion of iron into protoporphyrin. Biochim Biophys Acta. 1959 Feb;31(2):589–590. doi: 10.1016/0006-3002(59)90053-8. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N., CAUGHEY W. S. Porphyrin specificity of ferro:protoporphyrin chelatase from rat liver. Biochemistry. 1963 Mar-Apr;2:372–374. doi: 10.1021/bi00902a033. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N. Metal specificity of the ironprotoporphyrin chelating enzyme from rat liver. Biochim Biophys Acta. 1961 Sep 2;52:130–135. doi: 10.1016/0006-3002(61)90910-6. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., NISHIDA G. A new method of hemin isolation. Biochim Biophys Acta. 1957 Nov;26(2):437–437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mazanowska A. M., Neuberger A., Tait G. H. Effect of lipids and organic solvents on the enzymic formation of zinc protoporphyrin and haem. Biochem J. 1966 Jan;98(1):117–127. doi: 10.1042/bj0980117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYAMA H., SUGITA Y., YONEYAMA Y., YOSHIKAYA H. Stoichiometry of heme synthesis by partially purified enzyme preparation from duck erythrocytes. Biochim Biophys Acta. 1961 Feb 18;47:413–414. doi: 10.1016/0006-3002(61)90309-2. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 2. An in vestigation of the role offerrochelatase in the biosynthesis of various haem prosthetic groups. Biochem J. 1963 Apr;87:186–192. doi: 10.1042/bj0870186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., ROSS B. D. HAEM SYNTHASE AND COBALT PORPHYRIN SYNTHASE IN VARIOUS MICRO-ORGANISMS. Biochem J. 1965 Mar;94:557–562. doi: 10.1042/bj0940557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIETHMUELLER G., TUPPY H. HAEMSYNTHETASE (FERROCHELATASE) IN SACCHAROMYCES CEREVISIAE NACH AEROBEM UND ANAEROBEM WACHSTUM. Biochem Z. 1964 Sep 28;340:413–420. [PubMed] [Google Scholar]
- Sano S., Nanzyo N., Rimington C. Synthesis of porphyrin c-type compounds from protoporphyrinogen. Biochem J. 1964 Nov;93(2):270–280. doi: 10.1042/bj0930270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama Y., Tamai A., Yasuda T., Yoshikawa H. Iron-chelating enzyme from rat liver. Biochim Biophys Acta. 1965 Jul 29;105(1):100–105. doi: 10.1016/s0926-6593(65)80178-3. [DOI] [PubMed] [Google Scholar]


