Abstract
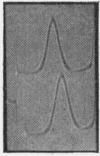
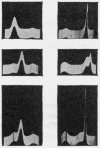
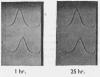
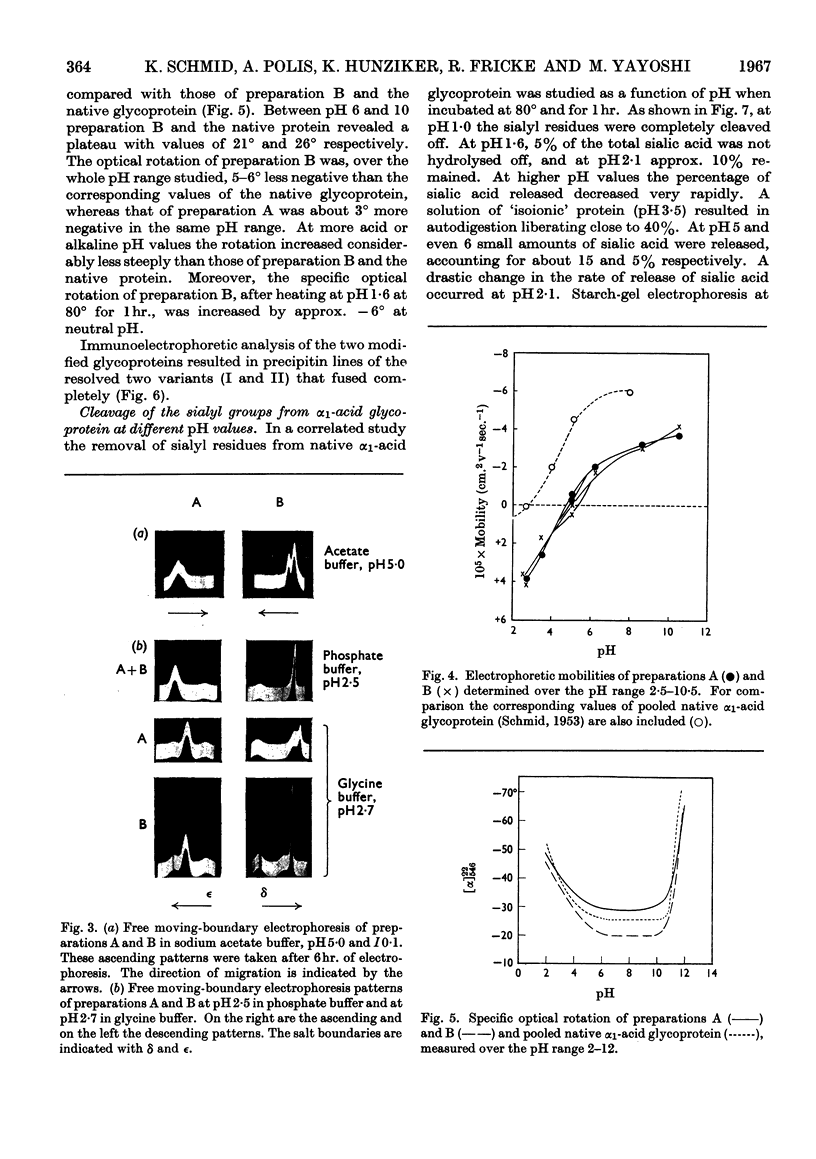
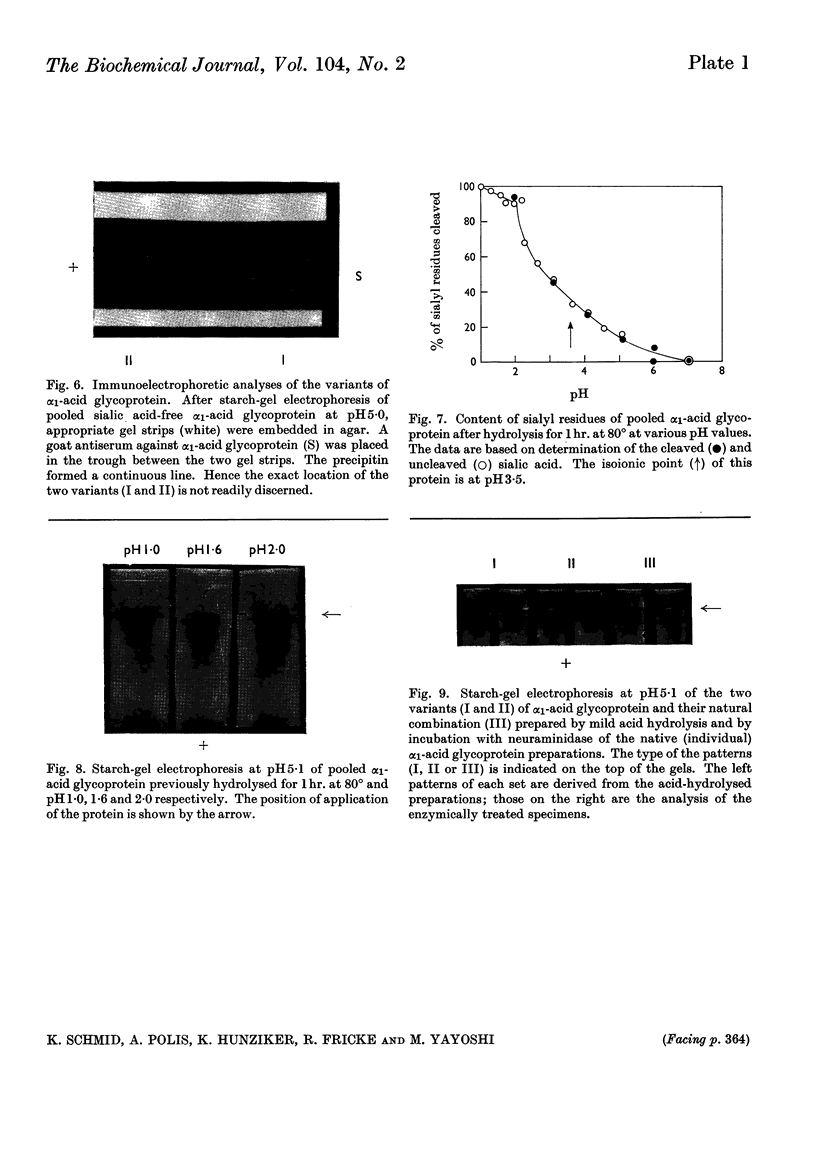
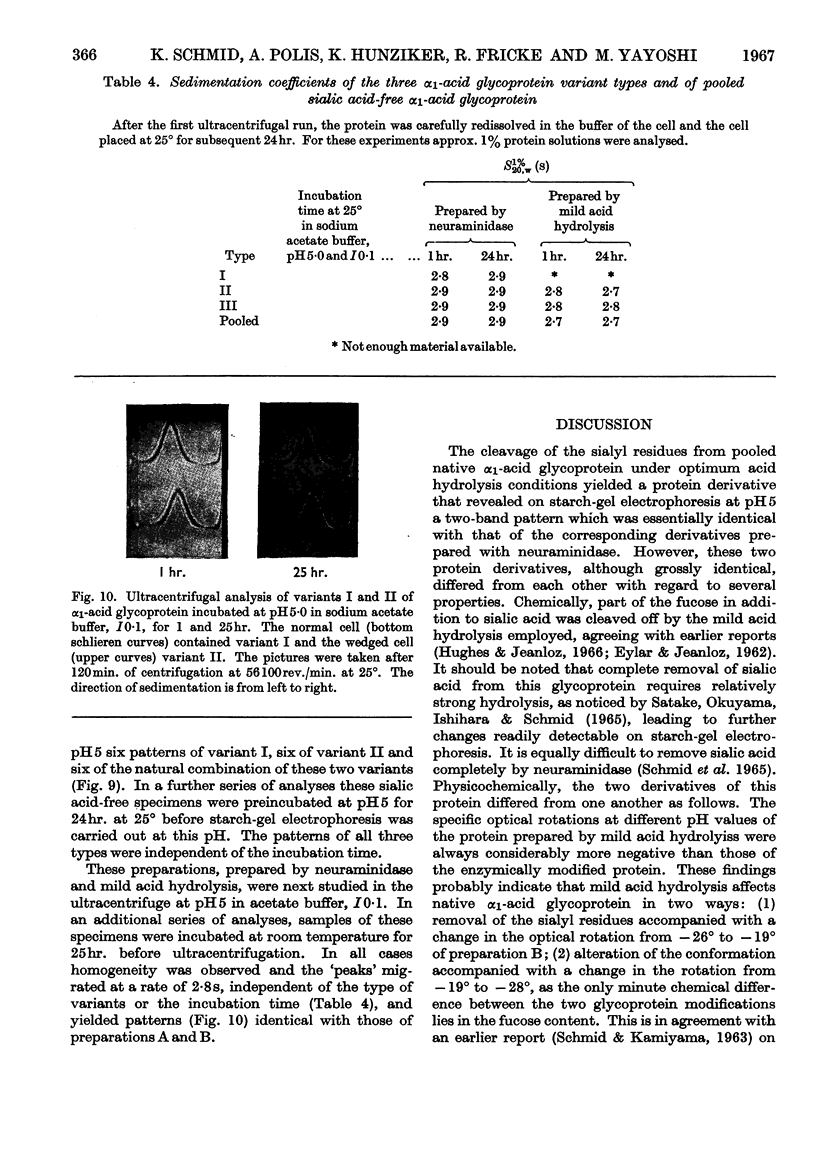
Some of the properties of sialic acid-free α1-acid glycoprotein prepared by mild acid hydrolysis (pH1·6 at 80° for 1hr.) were compared with those of neuraminidasetreated α1-acid glycoprotein. Chemically, the former contained less fucose (15%) and amide (2%) residues. Physicochemically, it had undergone certain changes primarily pertaining to the secondary structure, so that the specific optical rotation was more negative than that of the latter. A further expression of this change is probably the difference in the pH range of the resolution into two bands on electrophoresis. The resolution of the glycoprotein prepared by mild acid hydrolysis seems to be extended to more acidic pH values both by starch-gel and free moving-boundary electrophoresis. On ultracentrifugation both preparations appeared homogeneous and sedimented with a rate of 3s. Removal of sialyl residues at different pH values, in the range 1–7, showed that 2moles of sialic acid/mole of protein are very strongly bound. The two variants of α1-acid glycoprotein were isolated from pooled sialic acid-free α1-acid glycoprotein by preparative starch-gel electrophoresis and from selected blood of normal adults by fractionation by solubility and chromatography. Ultracentrifugal and starch-gel electrophoretic analyses at pH5, with incubation times of 1 or 24hr., demonstrated that no dissociation–association equilibrium (constant sedimentation coefficient and molecular weight) or isomerization (constant apparent electrophoretic mobilities) exist between the two variants. Therefore these variants are not sub-units of native α1-acid glycoprotein but represent modifications of naturally occurring proteins. Further, it was shown that the difference in the electrophoretic mobilities between the two variants was not due to any difference in amide content. Immunochemically, the two variants share the same determinants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI W., SCHMID K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem. 1961 Apr;236:1066–1074. [PubMed] [Google Scholar]
- CANFIELD R. E., ANFINSEN C. B. CHROMATOGRAPHY OF PEPSIN AND CHYMOTRYPSIN DIGESTS OF EGG WHITE LYSOZYME ON PHOSPHOCELLULOSE. J Biol Chem. 1963 Aug;238:2684–2690. [PubMed] [Google Scholar]
- Cann J. R. Multiple electrophoretic zones arising from protein-buffer interaction. Biochemistry. 1966 Mar;5(3):1108–1112. doi: 10.1021/bi00867a042. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Conway E. J., Byrne A. An absorption apparatus for the micro-determination of certain volatile substances: The micro-determination of ammonia. Biochem J. 1933;27(2):419–429. [PMC free article] [PubMed] [Google Scholar]
- DEBRAY-SACHS M., ANTOINE B., FINE J. M. [Immunological analysis of normal human serum after electrophoresis using starch gel]. Rev Fr Etud Clin Biol. 1961 May;6:435–445. [PubMed] [Google Scholar]
- GRABAR P. The use of immunochemical methods in studies on proteins. Adv Protein Chem. 1958;13:1–33. doi: 10.1016/s0065-3233(08)60597-5. [DOI] [PubMed] [Google Scholar]
- Hughes R. C., Jeanloz R. W. Sequential periodate oxidation of the slpha-acid glycoprotein of human plasma. Biochemistry. 1966 Jan;5(1):253–258. doi: 10.1021/bi00865a033. [DOI] [PubMed] [Google Scholar]
- IKENAKA T., GITLIN D., SCHMID K. PREPARATION AND CHARACTERIZATION OF THE LOW MOLECULAR WEIGHT HUMAN PLASMA 3S GAMMA-1-GLOBULINS. J Biol Chem. 1965 Jul;240:2868–2876. [PubMed] [Google Scholar]
- JOLLES P., JAUREGUI ADELL J., JOLLES J. LE LYSOZYME DE BLANC D'OEUF DE POULE: DISPOSITION DES PONTS DISULFURES. C R Hebd Seances Acad Sci. 1964 Apr 13;258:3926–3928. [PubMed] [Google Scholar]
- Katz E. P., Mechanic G. L., Glimcher M. J. The ultracentrifugal and free zone electrophoretic characterization of the neutral soluble proteins of embryonic bovine enamel. Biochim Biophys Acta. 1965 Oct 18;107(3):471–484. doi: 10.1016/0304-4165(65)90191-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARSHALL R. D., NEUBERGER A. Amide nitrogen content of ovomucoid. Nature. 1960 Apr 23;186:311–312. doi: 10.1038/186311a0. [DOI] [PubMed] [Google Scholar]
- POPENOE E. A., DREW R. M. The action of an enzyme of Clostridium perfringens on orosomucoid. J Biol Chem. 1957 Oct;228(2):673–683. [PubMed] [Google Scholar]
- Petersen H. A., Foster J. F. The microheterogeneity of plasma albumins. 3. Comparison of some physicochemical properties of subfractions. J Biol Chem. 1965 Oct;240(10):3858–3865. [PubMed] [Google Scholar]
- SATAKE M., OKUYAMA T., ISHIHARA K., SCHMID K. THE CARBOHYDRATE-POLYPEPTIDE LINKAGES, THE AMINO ACID SEQUENCES OF THE PEPTIDES ADJACENT TO SOME OF THESE BONDS, AND THE COMPOSITION AND SIZE OF THE CARBOHYDRATE UNITS OF ALPHA-1-ACID GLYCOPROTEIN. Biochem J. 1965 Jun;95:749–757. doi: 10.1042/bj0950749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID K., BINETTE J. P., TOKITA K., MOROZ L., YOSHIZAKI H. THE POLYMORPHIC FORMS OF ALPHA-1-ACID GLYCOPROTEIN OF NORMAL CAUCASIAN INDIVIDUALS. J Clin Invest. 1964 Dec;43:2347–2352. doi: 10.1172/JCI105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID K., BURGI W. Preparation and properties of the human plasma Ba-alpha2-glycoproteins. Biochim Biophys Acta. 1961 Mar 4;47:440–453. doi: 10.1016/0006-3002(61)90539-x. [DOI] [PubMed] [Google Scholar]
- SCHMID K., KAMIYAMA S. Studies on the structure of alpha1-acid glycoprotein. IV. Optical properties. Biochemistry. 1963 Mar-Apr;2:271–275. doi: 10.1021/bi00902a011. [DOI] [PubMed] [Google Scholar]
- SCHMID K., POLIS A. Electrophoretic behavior of normal human serum albumin at pH4.0. II. Interpretation of electrophoretic patterns in terms of a reversible protein interaction. J Biol Chem. 1960 May;235:1321–1325. [PubMed] [Google Scholar]
- SCHMID K., TOKITA K., YOSHIZAKI H. THE ALPHA-1-ACID GLYCOPROTEIN VARIANTS OF NORMAL CAUCASIAN AND JAPANESE INDIVIDUALS. J Clin Invest. 1965 Aug;44:1394–1401. doi: 10.1172/JCI105244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH E. L., BROWN D. M., WEIMER H. E., WINZLER R. J. Sedimentation, diffusion, and molecular weight of a mucoprotein from human plasma. J Biol Chem. 1950 Aug;185(2):569–575. [PubMed] [Google Scholar]
- SPIRO M. J., SPIRO R. G. Composition of the peptide portion of fetuin. J Biol Chem. 1962 May;237:1507–1510. [PubMed] [Google Scholar]
- Tokita K., Burke J. F., Yoshizaki H., Fischer S., Schmid K. The constancy of the alpha-1-acid glycoprotein variants of normal adults under conditions of severe stress. J Clin Invest. 1966 Oct;45(10):1624–1630. doi: 10.1172/JCI105469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WEIMER H. E., MEHL J. W., WINZLER R. J. Studies on the mucoproteins of human plasma. V. Isolation and characterization of a homogeneous mucoprotein. J Biol Chem. 1950 Aug;185(2):561–568. [PubMed] [Google Scholar]
- ZILLIKEN F., BRAUN G. A., GYORGY P. Gynaminic acid, a naturally occurring form of neuraminic acid in human milk. Arch Biochem Biophys. 1955 Feb;54(2):564–566. doi: 10.1016/0003-9861(55)90073-4. [DOI] [PubMed] [Google Scholar]