Abstract
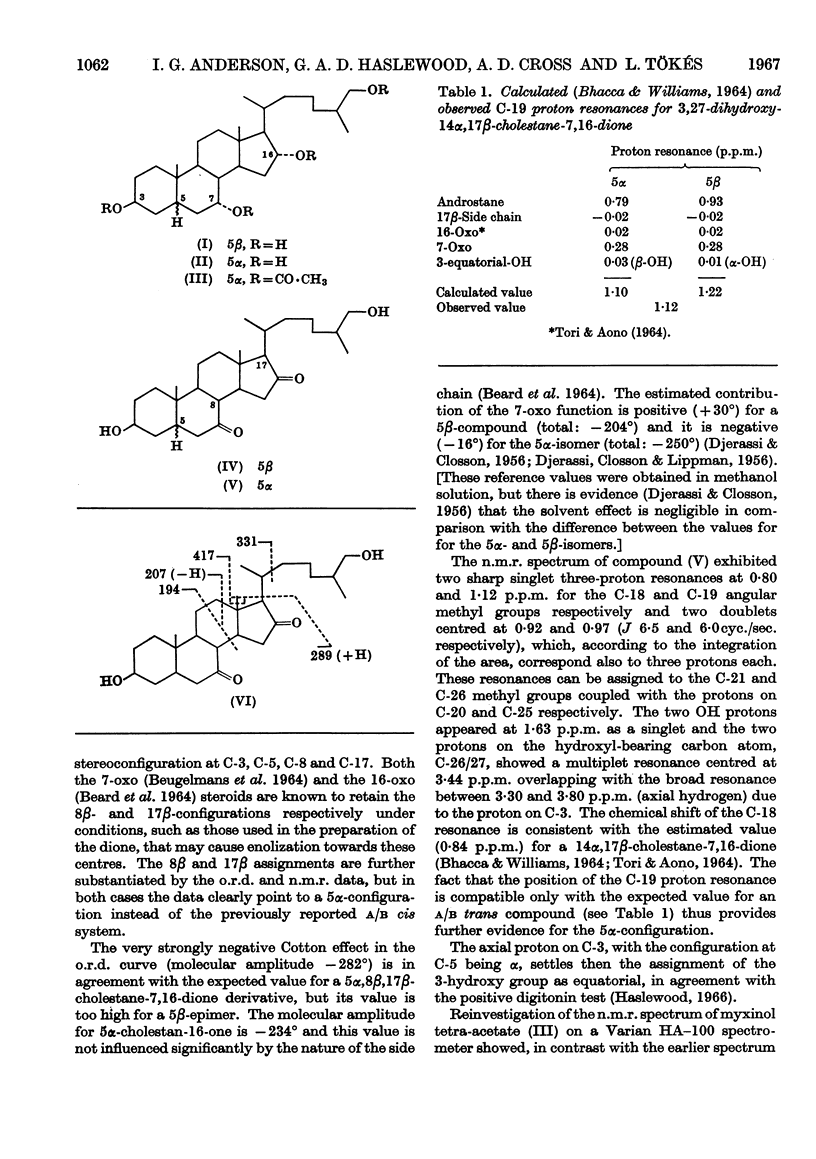
1. Preliminary spectroscopic examination of a second component of hagfish bile salts suggested that it might be 3β,7α,26(27)-trihydroxy-5α-cholestane. 2. Impure reduction products of the 3β,26(27)-dihydroxycholestane-7,16-dione previously made from myxinol disulphate appeared also to have the 5α-configuration. 3. Infrared, nuclear-magnetic-resonance and mass-spectrographic as well as optical-rotatory-dispersion measurements on 3β,26(27)-dihydroxycholestane-7,16-dione showed that it was a 5α-compound. 4. Myxinol is thus 3β,7α,16α,26(27)-tetrahydroxy-5α-cholestane; new nuclear-magnetic-resonance measurements on myxinol tetra-acetate at higher resolution confirm this structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cross A. D. Nuclear-magnetic-resonance and mass-spectral study of myxinol tetra-acetate. Biochem J. 1966 Jul;100(1):238–241. doi: 10.1042/bj1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslewood G. A. Comparative studies of bile salts, myxinol disulphate, the principal bile salt of hagfish (Myxinidae). Biochem J. 1966 Jul;100(1):233–237. doi: 10.1042/bj1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]


