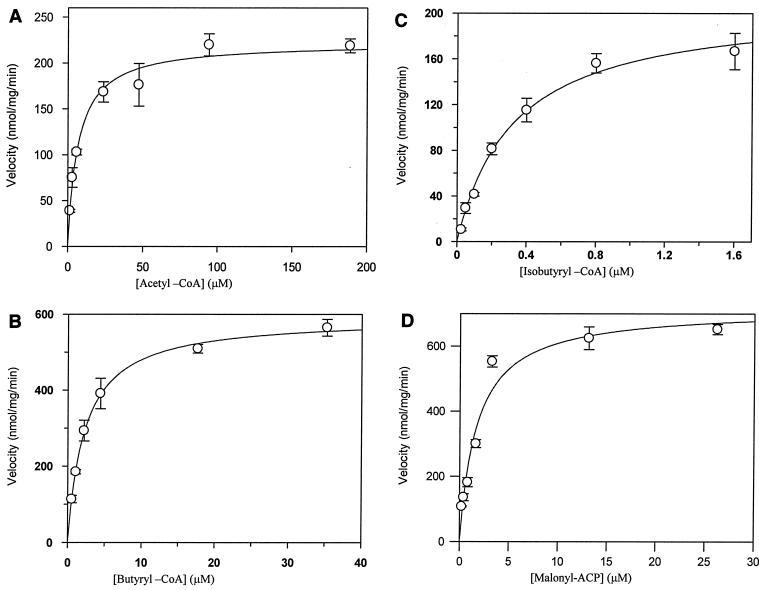
FIG. 7.
Kinetic analysis of saFabH for acetyl-CoA, butyryl-CoA, and MACP. The initial velocities of product formation were measured with purified saFabH and MACP in the presence of increasing concentrations of acetyl-CoA (A), butyryl-CoA (B), and isobutyryl-CoA (C). The initial velocities of product formation were measured with purified saFabH and butyryl-CoA in the presence of increasing concentrations of MACP (D). Each datum point is the average of three determinations.