Figure 3.
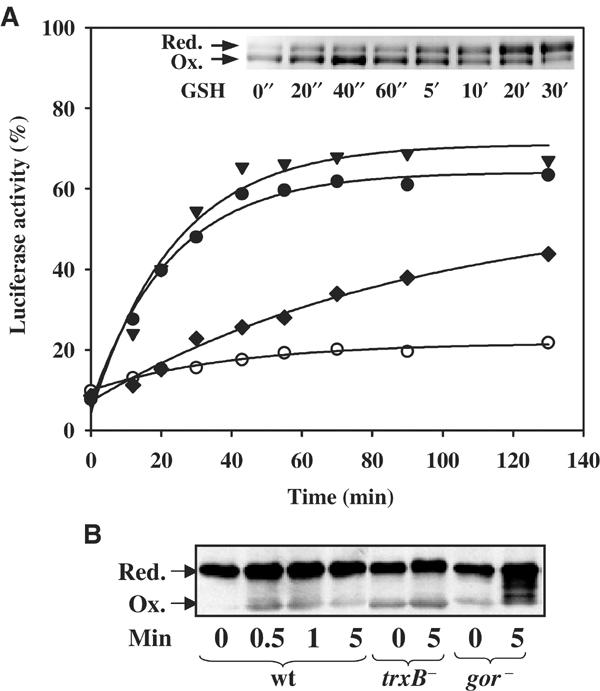
Identification of the physiological reductants of Hsp33. (A) Reduction of Hsp33 is catalyzed by thioredoxin in a TrxB- and NADPH-dependent manner in vitro. Luciferase (140 nM) was thermally unfolded in the presence of DnaK/DnaJ/GrpE (molar ratios 5:2:5) and a five-fold molar excess of active Hsp33 dimer for 8 min at 43°C. After 12 min of incubation at room temperature, the reaction was shifted to 30°C (t=0 min) and the incubation reaction was continued in the (○) absence of any additives or in the presence of either (▾) 5 mM DTT, (⧫) 5 mM GSH or (•) 2.8 μM TrxA, 70 nM TrxB, 50 μM NADPH. Only the recovery period at 30°C is shown. Inset: Physiological concentrations of reduced glutathione are not capable of quickly reducing oxidized Hsp33 dimers. Oxidized Hsp33 dimers (3 μM) were incubated in the presence of 5 mM GSH at 30°C. At the time points indicated, aliquots were removed and thiol trapping with AMS was performed as described. (B) Glutaredoxin and thioredoxin systems reduce Hsp33 in vivo. DHB4 wild-type, WM93 (ΔtrxB) or WP840 (Δgor) cells were grown at 30°C until an OD600 of 0.5 was reached. Then, the cells were shifted to 45°C. At 1 min after the shift, a sample was taken (t=0) and H2O2 was added (4 mM). Further samples were taken at the indicated time points. In vivo thiol trapping with AMS was performed and the samples were processed as described.
