Figure 4.
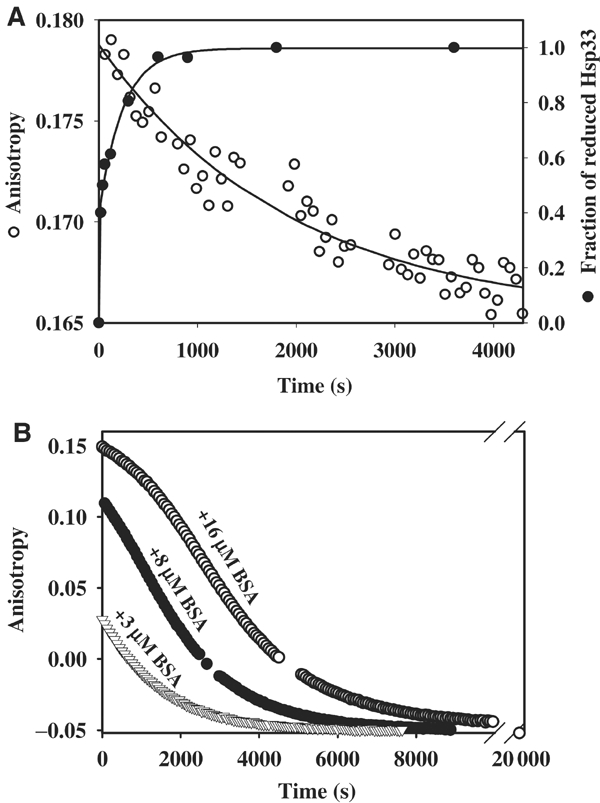
Identification of a stable Hsp33 intermediate: the reduced, active Hsp33 dimer. (A) Reduced Hsp33 dimers are kinetically stable. Oxidized Hsp33 dimers (3 μM) were incubated in 40 mM HEPES-KOH, 20 mM KCl, pH 8.0, at 20°C. A stable anisotropy signal at λex=280 nm and λem=350 nm was detected. To monitor (○) dissociation of reduced Hsp33 dimers, 5 mM DTT was added to the reaction. The anisotropy signal decreased and approached the lower signal of monomeric, reduced Hsp33. The curve was fitted according to a pseudo-first-order reaction and a rate constant of 5.2 × 10−4 s−1 was determined. A very similar rate constant was obtained when 15 μM Hsp33 dimers were used instead (4.7 × 10−4 s−1). To monitor the (•) reduction of Hsp33's thiol groups under these conditions, aliquots were taken and AMS trapped as described. The proteins were separated on 14% SDS–PAGE and the fraction of reduced Hsp33 protein was determined using a densitometer. A rate constant of 0.018 s−1 was determined for the reduction process of Hsp33. (B) Substrate proteins stabilize reduced Hsp33 dimers and delay inactivation of Hsp33. Oxidized Hsp33 dimers (3 μM) labeled with the fluorescent dye Oregon-green were incubated in 40 mM HEPES-KOH, 20 mM KCl, pH 8.0, at 20°C in the presence of (▿) 3 μM BSA, (•) 8 μM BSA or (○) 16 μM BSA. To reduce the dimeric Hsp33–BSA complexes, 5 mM DTT was added to the reaction, and the change in anisotropy signal was followed at λex=506 nm and λem=524 nm. Thiol trapping experiments showed that the rate of Hsp33 reduction was not significantly influenced by the presence of 16 μM BSA. Analysis of the apparent dissociation rate of fluorescently labeled Hsp33 dimers in the absence of BSA revealed a rate constant that was very similar to unlabeled Hsp33 (5.6 × 10−4 s−1).
