Figure 4.
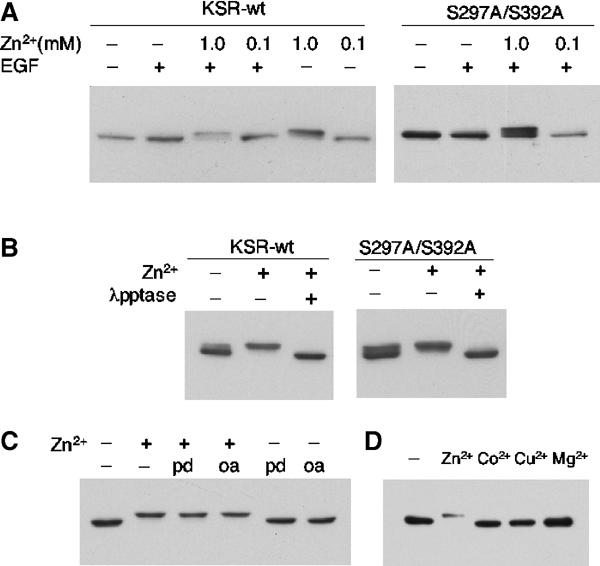
Elevated Zn2+ levels drastically increase the phosphorylation of KSR in HEK293 cells. (A) Addition of 1 mM Zn2+ causes a mobility shift of KSR. Flag-tagged KSR1 was transfected into HEK293 cells. At 24 h after transfection, cell cultures were treated with ZnSO4 for 3 h and/or 50 ng/ml EGF for 3 min. The mouse KSR protein in the cell lysates was analyzed by SDS–PAGE and Western blot using anti-M2 antibody. In a control test, a Flag-tagged Smad3 protein displayed no shift under the same condition (data not shown). On the right panel, both serine 297 and 392 were changed to alanine by in vitro mutagenesis. (B) In vitro treatment of lambda phosphatase eliminated the up-shift of KSR, indicating that the Zn2+-induced shift of the protein is due to phosphorylation. (C) Phosphatase inhibitors did not mimic or cause additional up-shifting of KSR mobility. PD, PD98059. OA, Okadatic acid. (D) Addition of 1 mM CoCl2, CuSO4 or MgCl2 does not cause the up-shift of KSR mobility.
