Figure 3.
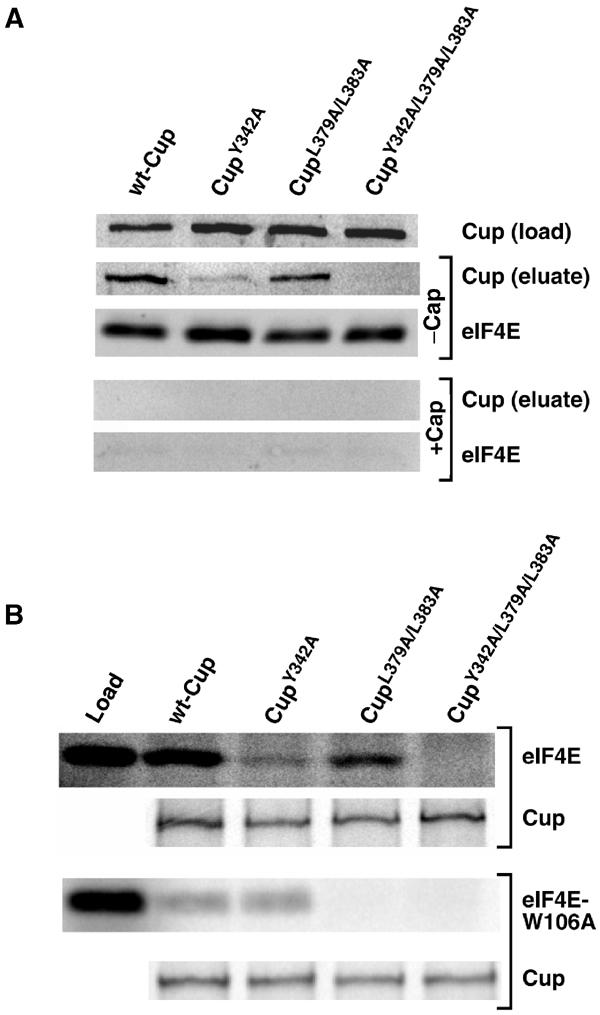
Cup contains two eIF4E-binding sites. (A) Drosophila S2 cells were transfected with plasmids expressing protein A-tagged wild-type and mutant forms of Cup. Extracts prepared from transfected cells were mixed with 7m-GTP-sepharose with and without soluble 7m-GDP (cap). Western blots to detect the indicated Cup proteins in the total cell extracts, which represent 15% of protein used in the immunoprecipitations, as well as Cup and eIF4E in the eluates from captures are shown. (B) Labeled eIF4E or eIF4E-W106A as well as wild-type and mutant FLAG-tagged Cup proteins were generated via coupled transcription/translation in rabbit reticulocyte lysate in the presence of 35S-methionine. The eIF4E or eIF4E-W106A was mixed with each of the Cup proteins and immunoprecipitated using an anti-FLAG antibody and protein G agarose. A sample of the in vitro-translated eIF4E (load) representing 50% of protein used in the captures and the elutions from the immunoprecipitations of the indicated Cup proteins are shown.
