Figure 2.
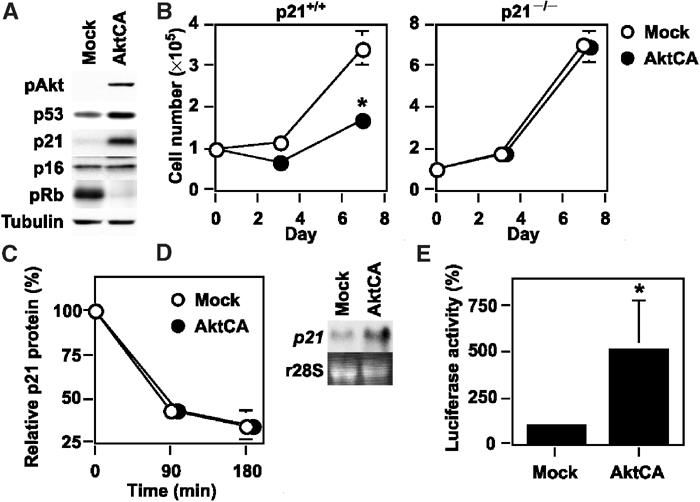
Upregulation of p21 is essential for Akt-induced growth arrest. (A) Whole-cell lysates (30 μg) of pLNCX (Mock)- or AktCA-infected endothelial cells on day 0 were examined for the expression of phospho-Akt (pAkt), cell cycle regulatory proteins and tubulin (loading control) by Western blotting. (B) MEF derived from wild-type (p21+/+) or p21-deficient mice (p21−/−) were infected with pLNCX (Mock) or AktCA, purified with G418 for 7 days and seeded at a density of 1 × 105 cells per 100 mm plate on day 0. Cell number per 100 mm plate was then counted at indicated time points. *P<0.001 versus Mock, ANOVA, n=4. (C) Human endothelial cells infected with pLNCX (Mock) or AktCA were treated with cycloheximide (10 μg/ml) for the indicated time interval. Whole-cell lysates (30 μg) were then prepared at each time point and assayed for the expression of p21 and actin (loading control) by Western blotting. The graph indicates the results of densitometric analysis for the levels of p21 protein relative to actin expression. The value at time 0 is set at 100%. (D) Total RNA (30 μg) was extracted from human endothelial cells infected with pLNCX (Mock) or AktCA and analyzed for p21 mRNA levels by Northern blotting (upper panel). Ribosomal RNA was used as an internal control (lower panel). (E) The luciferase reporter gene plasmid controlled by the promoter of the human p21 gene was transfected into endothelial cells infected with pLNCX (Mock) or AktCA 24 h before the luciferase activity was measured. The activity in mock-infected cells is set at 100%. *P<0.05 versus Mock, paired t-test, n=4.
