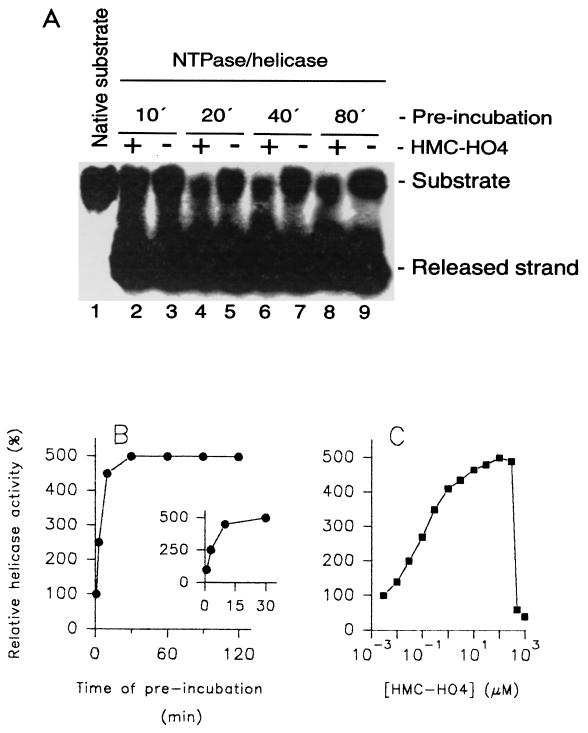
FIG. 4.
Modulation of the helicase activity of HCV NTPase/helicase by HMC-HO4. (A and B) Investigation of the helicase activity of HCV NTPase-helicase as a function of the time of duration of the interaction of HMC-HO4 with the DNA substrate. Aliquots of the DNA substrate (lane 1) were incubated in the presence of 150 μM HMC-HO4 or in the absence of the compound. At the indicated time points, 10 min (lanes 2 and 3), 20 min (lanes 4 and 5), 40 min (lanes 6 and 7), and 80 min (lanes 8 and 9), the unwinding reaction was started by addition of the enzyme or the enzyme and HMC-HO4, as described in Materials and Methods. The samples were separated in a TBE-polyacrylamide gel and visualized by exposure of the dried gel to X-ray film for 20 h (A). For determination of the activity of the enzyme, the parts of the gels corresponding to the released strand were excised and the 32P radioactivity was quantified as described in Materials and Methods. The unwinding activity of the enzyme measured without the preincubated substrate was referred to as 100% (B). The inset demonstrates an expanded view of the region from 0 to 30 min of preincubation. (C) Effect of preincubation of the DNA substrate with HMC-HO4 on the activities of HCV NTPase/helicase as a function of increasing concentrations of the compound. Aliquots of the DNA substrate were incubated in the presence of increasing concentrations of HMC-HO4 or in the absence of the compound for 20 min. The reaction was started by addition of the enzyme, and the unwinding activity was determined as described above. The results shown are representative of those for three independent experiments.