Figure 1.
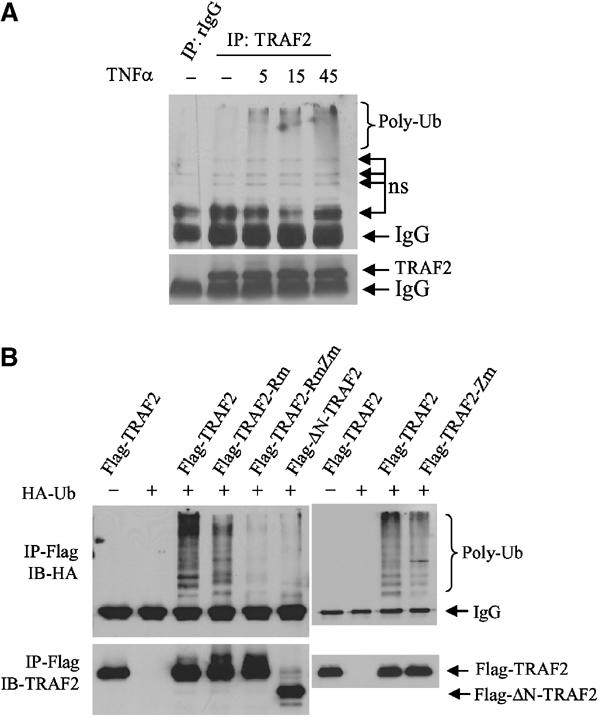
Intact RING and zinc finger domains of TRAF2 are required for its ubiquitination in vivo. (A) In vivo ubiquitination of endogenous TRAF2. HeLa cells were treated with hTNFα (40 ng/ml) at indicated time points, and proteins were extracted with RIPA buffer (supplemented with 20 mM NEM and 5 mM ubiquitin aldehyde). Protein extracts were then subjected to immunoprecipitation with anti-TRAF2 Ab or control rabbit IgG, followed by immunoblot analysis with the aid of anti-ubiquitin Ab (upper panel; ns: nonspecific bands). The same membrane was stripped and reprobed with anti-TRAF2 Ab (lower panel). (B) Intact RING and zinc finger domains of TRAF2 are required for its ubiquitination in vivo. Flag-TRAF2 (1 μg), Flag-TRAF2-Rm (1 μg, C49A, H51A, C54A and C57A), Flag-TRAF2-RmZm (1 μg, C49A, H51A, C209A and C212A) and Flag-ΔN-TRAF2 (1 μg, Δ1–87 amino acids from N-terminal) were co-transfected with HA-Ub (2 μg) to HeLa cells. At 36 h after transfection, proteins were extracted with 2% SDS/TBS, diluted with 9 vol 1% Triton X-100/TBS and subjected to immunoprecipitation (IP) using anti-Flag Ab and Western blot analysis using anti-HA Ab. The same membrane was stripped and reprobed with anti-TRAF2 polyclonal Ab (lower panel).
