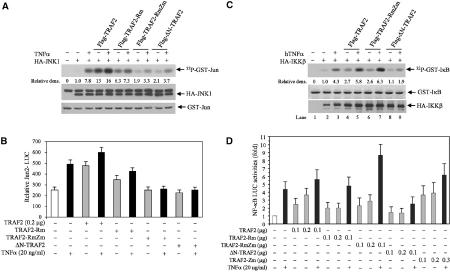
Figure 2.
Intact RING and zinc finger domains of TRAF2 are required for activation of JNK but not of NF-κB. (A) TRAF2-RmZm and ΔN-TRAF2 inhibit TNFα-induced JNK activation. HA-JNK1 (0.5 μg) was co-transfected with wt or mutant forms of TRAF2 (1.0 μg) in HeLa cells. At 36 h after transfection, cells were treated with or without hTNFα (40 ng/ml) for 15 min before proteins were extracted with TNE buffer. HA-JNK1 was then immunopurified with anti-HA Ab and subjected to in vitro kinase reaction using GST-c-Jun1–87 as a substrate. Reaction mixtures were incubated at 30°C for 20 min followed by separation on SDS–PAGE, transfer onto nitrocellulose membrane and autoradiography to detect GST-c-Jun1–87 phosphorylation. The same membrane was blotted to detect the HA-JNK1 level (middle panel) using anti-HA Ab and stained with Ponceau S to monitor GST-c-Jun1–87 level (lower panel). Relative density values reflect quantification of corresponding bands by phosphorimager. (B) TRAF2-RmZm and ΔN-TRAF2 inhibit TNFα-induced c-Jun transcriptional activities. HeLa cells cultured on six-well plates were transfected with Jun2-LUC (0.2 μg), β-gal (0.1 μg), and wt or mutant Flag-TRAF2 (0.2 μg). After 36 h, cells were treated with or without hTNFα (10 ng/ml) for 6 h. Luciferase activities were then measured using a luciferase assay system (Promega) and values were normalized based on β-gal activities. The data represent triplicate experiments. (C) Whereas ΔN-TRAF2 inhibits TNFα-induced IKK activation, TRAF2-RmZm does not. HA-IKKβ (1.0 μg) was co-transfected with wt or mutant forms of TRAF2 (1.0 μg) in HeLa cells. At 36 h after transfection, cells were treated with or without hTNFα (40 ng/ml) for 5 min before proteins were extracted with TNE buffer. HA-IKKβ was then immunopurified with anti-HA Ab and subjected to in vitro kinase reaction using GST-IκB1–55 as a substrate. Reaction mixtures were incubated at 30°C for 20 min followed by separation on SDS–PAGE, transfer onto a nitrocellulose membrane and autoradiography to detect GST-IκB1–55 phosphorylation. The same membrane was blotted for HA-IKKβ level (lower panel) using anti-HA Ab and stained with Ponceau S to detect GST-IκB1–55 level (middle panel). (D) The intact TRAF2 RING domain is not required for TNFα-induced NF-κB activities. HeLa cells cultured on six-well plates were transfected with NF-κB-LUC (0.2 μg), β-gal (0.1 μg), and wt or mutant forms of TRAF2 (0.2 μg), and luciferase activities of NF-κB-Luc were analyzed as described in (B). Data represent triplicate experiments.