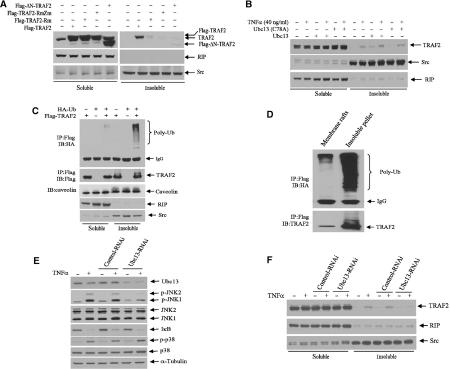
Figure 4.
Ubc13 and an intact RING domain are required for TRAF2 translocation to the insoluble fraction. (A) TRAF2 translocation to the insoluble fraction requires its intact RING domain. Wt or mutant forms of TRAF2 were expressed in HeLa cells, and 36 h later nonionic detergent soluble and insoluble fractions were prepared as described in Materials and methods. Both transfected and endogenous TRAF2 levels were analyzed by Western blot with anti-TRAF2 Ab. The same membranes were stripped and reprobed for anti-RIP1 and anti-Src antibodies for control of fractionation. (B) Translocation of endogenous TRAF2 to the insoluble fraction is induced by Ubc13. HeLa cells were transfected with Ubc13 (2.0 μg) or Ubc13mut. After 36 h, cells were treated with or without hTNFα (40 ng/ml) for 15 min, and nonionic detergent soluble and insoluble fractions were prepared as described in Materials and methods. The distribution of endogenous TRAF2, RIP1 and Src proteins was analyzed by immunoblotting using the corresponding antibodies. (C) Polyubiquitinated TRAF2 is located within the insoluble fraction. HeLa cells were co-transfected with Flag-TRAF2 (1.0 μg) and HA-Ub (2.0 μg). After 36 h, soluble and insoluble fractions were prepared as described in Materials and methods, and protein samples were subjected to immunoprecipitation with anti-Flag Ab followed by immunoblot analysis with anti-HA Ab to detect ubiquitinated TRAF2. The same membrane was reprobed for TRAF2 level with anti-TRAF2 Ab. The same protein samples were separated on another SDS–PAGE, and distributions of caveolin-1, RIP1 and Src were analyzed with corresponding antibodies. (D) Polyubiquitinated TRAF2 is localized both in membrane rafts and in the insoluble pellet. HeLa cells (3 × 100 mm plates) were co-transfected with Flag-TRAF2 (1.0 μg) and HA-Ub (2.0 μg). After 36 h, cells were lysed in TNPN buffer (containing 20 mM NEM) on ice for 20 min, and membrane rafts and the insoluble pellet were prepared as described in Materials and methods. Protein samples were then subjected to immunoprecipitation with anti-Flag Ab followed by immunoblot analysis with anti-HA Ab to detect the distribution of ubiquitinated TRAF2. The same membrane was reprobed to determine TRAF2 levels with anti-TRAF2 Ab. (E) Inhibition of Ubc13 expression inhibits TNFα-induced JNK activation. HeLa cells were infected with 5 ml of high-titer retroviral supernatants of pRS (control) or pRS-Ubc13 in the presence of 4 μg/ml polybrene overnight followed by further incubation in fresh medium for 72 h before exposure to hTNFα (40 ng/ml) for 10 min. Protein samples were extracted in TNE buffer (containing phosphatase inhibitors) and subjected to immunoblot analysis with anti-Ubc13, anti-phospho-JNK, anti-phospho-p38, anti-IκB and control antibodies as indicated. (F) Inhibition of Ubc13 expression inhibits TNFα-induced TRAF2 translocation to insoluble fraction. HeLa cells were infected with retroviral supernatants as in (E). Cells were treated with or without hTNFα (40 ng/ml) for 10 min, and soluble and insoluble fractions were extracted. Protein samples were then subjected to immunoblot analysis using anti-TRAF2, anti-RIP1 and anti-Src antibodies.