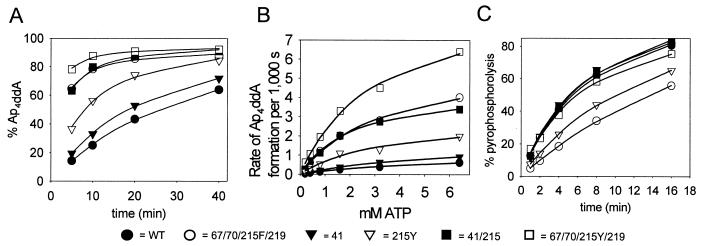
FIG. 2.
Effect of AZT resistance mutations on removal of [32P]ddAMP from blocked primer-template through transfer to ATP or PPi. (A) [32P]ddAMP-terminated L32 primer-WL50 template (5 nM) was incubated with the indicated RT and 3.2 mM ATP for 5, 10, 20, or 40 min at 37°C. Products were separated on a 20% denaturing polyacrylamide gel (data not shown). The radioactivity in primer and Ap4ddA was quantitated by phosphorimaging, and the percent Ap4ddA formed was plotted against time. The lines represent the best fit to the Michaelis-Menten equation using Sigmaplot 4.0. (B) Ap4ddA synthesis as a function of ATP concentration. Reactions were carried out as for panel A but with 0.2 to 6.4 mM ATP and for 1 to 10 min, depending on the enzyme, to allow a maximum of 35% Ap4ddA formation. The rate of Ap4ddA formation was calculated and plotted against time. (C) PPi-dependent formation of [32P]ddATP (pyrophosphorolysis). Experiments were performed as described for panel A but with 5 μM PPi instead of ATP. The amount of radioactivity in primer and ddATP was quantitated by phosphorimaging, and the percentage of label in ddATP was plotted against time.