Abstract
Six strains of staphylococci were exposed to levofloxacin, moxifloxacin, or trovafloxacin in an in vitro pharmacodynamic model under both aerobic and anaerobic conditions. Each agent demonstrated a rapid 3-log10 kill versus susceptible isolates regardless of condition. Against clinical isolates with reduced susceptibility, regrowth occurred by 24 h and was frequently associated with further increases in MICs.
When first introduced into clinical practice, ciprofloxacin appeared to have good activity against methicillin-susceptible and -resistant staphylococci, with MICs ranging from 0.25 to 2 mg/liter (7). However, within 2 years, most strains of methicillin-resistant Staphylococcus aureus had developed resistance to ciprofloxacin, with MICs ranging from 4 to 32 mg/liter (7). Newer, more lipophilic fluoroquinolones, including levofloxacin, trovafloxacin, and moxifloxacin, may not be eliminated as efficiently from bacterial cells by efflux pumps as to older, more hydrophilic fluoroquinolones. These agents also possess greater affinity for secondary topoisomerase targets and may be less likely to foster bacterial resistance (2).
Studies evaluating the effect of anaerobiosis on the activity of fluoroquinolones have not been in universal agreement. Lewin et al. found that older fluoroquinolones were bacteriostatic against Escherichia coli and S. aureus under strict anaerobic conditions and suggested this may be due to impaired uptake of drug into bacterial cells in an anaerobic environment (3, 5). Conversely, Wise et al. found sparfloxacin and ciprofloxacin were bactericidal against E. coli under anaerobic conditions (1). Our group found five older fluoroquinolones to be bactericidal against staphylococci, regardless of whether studied in an aerobic or anaerobic environment, although the rate of kill appeared slower anaerobically (11).
Because staphyloccocci are facultative organisms and may cause infections in anaerobic and microaerophilic environments, understanding the influence of environment on fluoroquinolone activity is important. As an example, these agents may be considered by clinicians as adjunctive to surgery in the treatment of serious soft tissue abscesses. The purposes of this investigation were to study the impact of the aerobic versus anaerobic environment on the activity of clinically relevant concentrations of the newer fluoroquinolones moxifloxacin (400-mg dose), trovafloxacin (200-mg dose), and levofloxacin (500-mg dose) against methicillin-susceptible and -resistant S. aureus (MSSA and MRSA, respectively) and Staphylococcus epidermidis (MSSE and MRSE, respectively) by using an in vitro pharmacodynamic model (IVPDM) and to examine changes in antibiotic susceptibility over time by measuring pre- and post-antibiotic exposure MICs.
(This study was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif.)
The IVPDM used in this investigation has been previously described by Zabinski et al. (10). Levofloxacin (Ortho-McNeil Pharmaceutical, Raritan, N.J.), trovafloxacin (Pfizer, New York, N.Y.), or moxifloxacin (Bayer, West Haven, Conn.) stock solution was injected into the system as a bolus to attain a desired starting peak concentration. Antibiotic-free medium (cation-supplemented Mueller-Hinton broth [CSMHB]) was pumped into the system, and an equal volume of antibiotic-containing medium was displaced into a waste vessel, resulting in the simulation of a first-order, one-compartment pharmacokinetic process. All experiments were completed in duplicate. The peak quinolone concentration and half-life simulated human pharmacokinetics, respectively, were as follows for levofloxacin, moxifloxacin, and trovafloxacin: 6 mg/liter and 8 h; 4 mg/liter and 12 h; and 2 mg/liter and 10 h. The initial inoculum in each experiment was 106 CFU/ml, and the temperature was maintained at 37°C. Ten 1-ml samples were drawn over 24 h at the following times: time zero; 30 min; and 1, 2, 3, 4, 6, 8, 12, and 24 h following antibiotic exposure. The potential for antibiotic carryover was addressed through the use of 1 g of polymeric binding resin (Amberlite XAD-4/1090; Rohm & Haas, Philadelphia, Pa.) per ml of broth and by serial dilution (9). Trypticase soy blood agar (Dimed, St. Paul, Minn.) plates were used for viable colony count determinations. After incubation for 18 to 24 h at 37°C, the numbers of CFU on each plate were counted. Aerobic experiments were conducted in ambient air, while an anaerobic environment was created by placing the pharmacodynamic system within a Bactron IV anaerobic chamber (Sheldon Manufacturing, Cornelius, Oreg.). The medium was prereduced before being inoculated with organisms in the anaerobic experiments.
The organisms tested included two MRSA isolates, ATCC 33592 and wild-type strain 87; one MSSA isolate, ATCC 29213; two MRSE isolates, ATCC 51625 and wild-type strain 126; and one MSSE isolate, ATCC 12228. Each organism was tested against each antibiotic under both aerobic and anaerobic conditions.
MICs for both unexposed organisms and those that had been exposed to antibiotics for 24 h in the IVPDM were determined under aerobic conditions in CSMHB by microtiter broth dilution at an inoculum of approximately 106 CFU/ml (6).
Reversed-phase high-pressure liquid chromatography was used to validate drug concentrations (8). Assays were linear between 0.31625 and 9 μg/ml (R2, ≥0.99). The R2 of the standard curves, the coefficients of variation of standards, and the coefficients of variation of the unknown analyses based upon an internal standard, respectively, were as follows: trovafloxacin, 0.993, 9.48%, and 7.52%; moxifloxacin, 0.994, 5.88%, and 5.36%; and levofloxacin, 0.996, 12.39%, and 11.56%.
Time-kill curves were plotted as declines in log10 CFU per milliliter versus time. Three-log kill was determined by visual inspection of the time-kill curve. The area under the kill curve (AUBKC) and the area under the kill times time curve (AUBKTC) were calculated by the trapezoidal rule, and a mean survival time (MST) was calculated by the equation AUBKTC/AUBKC (4). This measure of effect allows for a simple comparison of fluoroquinolone activity under aerobic and anaerobic conditions. MSTs among the experiments were compared by one-way analysis of variance with Tukey's post test by using GraphPad Prism version 3.02 for Windows (GraphPad Software, San Diego, Calif.; www.graphpad.com). Significance was defined as P < 0.05.
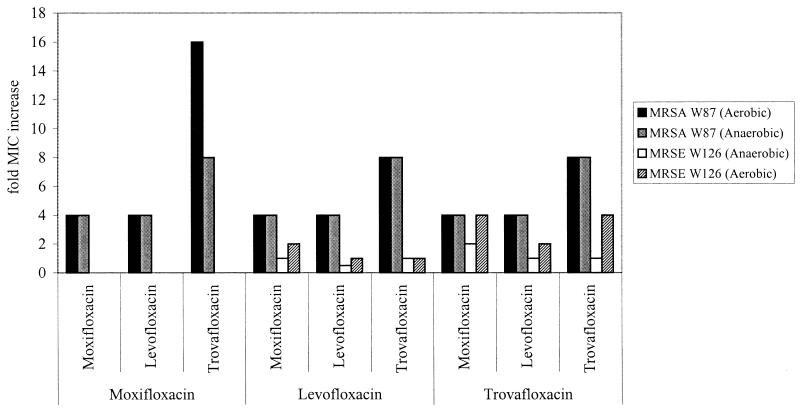
Preexposure MICs are shown in Table 1. None of the American Type Culture Collection (ATCC) isolates exhibited increased MICs after 24 h of antibiotic exposure. The MIC for S. aureus W87 determined at 24 h demonstrated a 4- to 16-fold increase after exposure to any of the three quinolones in the in vitro model, while S. epidermidis W126 demonstrated a 4-fold increase in MIC only after exposure to trovafloxacin (Fig. 1). Given that S. aureus W87 and S. epidermidis W126 were not susceptible to levofloxacin, decreases in susceptibility, not seen against the susceptible ATCC strains, were not unexpected. However, increased resistance was found after only 24 h of exposure to the drug, highlighting the fact that, against some strains, these agents not only may fail, but also may lead to high-level resistance.
TABLE 1.
MICs and MBCs for bacteria before antibiotic exposure
| Bacteria | Pre-antibiotic exposure MIC or MBC (mg/liter)
|
||
|---|---|---|---|
| Levofloxacin | Trovafloxacin | Moxifloxacin | |
| MSSA ATCC 29213 | 0.25 | 0.03 | 0.06 |
| MRSA ATCC 33592 | 0.25 | 0.03 | 0.06 |
| MRSA W87 | 8 | 2 | 2 |
| MSSE ATCC 12228 | 0.25 | 0.06 | 0.12 |
| MRSE ATCC 51625 | 0.25 | 0.03 | 0.06 |
| MRSE W126 | 4 | 2 | 1 |
FIG. 1.
Fold increases in MICs of wild-type MRSA and MRSE to moxifloxacin, levofloxacin, and trovafloxacin after exposure to moxifloxacin, levofloxacin, or trovafloxacin. (For example, after MRSA W87 was exposed to levofloxacin for 24 h in an aerobic environment, regrowth was associated with a fourfold increase in the MICs of moxifloxacin and levofloxacin and an eightfold increase in the MIC of trovafloxacin.) No S. epidermidis W126 isolates were recovered at 24 h from in vitro experiments with moxifloxacin.
Currently, susceptibility breakpoints for moxifloxacin or trovafloxacin against S. aureus or S. epidermidis do not exist. These clinical isolates may have had previous exposure to fluoroquinolones, resulting in the acquisition of resistance determinants and subsequently acquired further resistance traits in the model, resulting in high-level resistance. Also, resistant subpopulations in the inoculum, not detected by MIC testing, may have been present at the start of the in vitro experiments. Despite greater potency for gram-positive bacteria, these data suggest newer fluoroquinolones could lead to resistance in staphylococci.
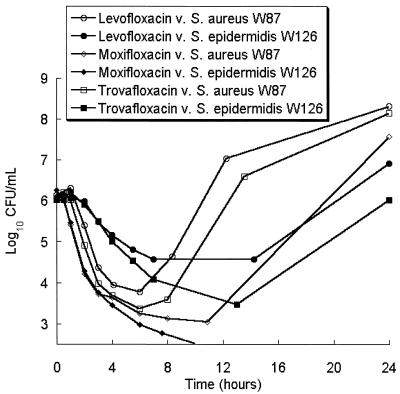
Each of the antibiotics achieved a 3-log reduction of the bacterial inoculum against each of the ATCC strains within 12 h, regardless of the environment, and regrowth was not observed (data not shown). All of the fluoroquinolones tested had, in general, poor activity against clinical isolates of MRSA and MRSE in an aerobic environment (Fig. 2); activity against these strains was similar in an anaerobic environment.
FIG. 2.
Activities of levofloxacin, moxifloxacin, and trovafloxacin against S. aureus W87 and S. epidermidis W126.
We attempted to detect differences in MST between experiments done in either an aerobic or anaerobic environment given a specific strain and antibiotic. Statistically significant differences were detected on two occasions, both involving moxifloxacin: against MSSA ATCC 29212, the MSTs (mean ± standard deviation of two experiments) were 13.3 ± 2.8 (anaerobic) and 67.2 ± 12.1 (aerobic) min (P < 0.001); against MRSE ATCC 51625, the MSTs were 6.2 ±2.7 (aerobic) and 38.7 ± 0.2 (anaerobic) min (P < 0.01). However, in both of these instances, bactericidal activity was apparent, because a 3-log kill had taken place within 10 h. Together, these data indicate that, in some instances, kill may be slightly altered by anaerobiosis, although the kill rate is not uniformly faster or slower in the anaerobic environment. The extent of kill, as demonstrated by 3-log kill, did not appear dependent on the presence of oxygen.
In that each of the fluoroquinolones in this study showed bactericidal activity and prevented regrowth under aerobic and anaerobic conditions versus susceptible bacteria, the results of this study are in agreement with those of our previous work with older fluoroquinolones. (11) In our previous work, while each of the agents was bactericidal, there was a consistent delay in activity with anaerobiosis. In this study, on only two occasions was a difference found between aerobic and anaerobic activity within a specific quinolone, and both of these involved moxifloxacin. In one of these cases, the activity was slightly greater under anaerobic conditions, although the rate of kill was also quite rapid under aerobic conditions.
Despite an initial kill, isolates with reduced initial fluoroquinolone susceptibility demonstrated measurable changes in MIC pre- versus post-antibiotic exposure. Although the poor performance against isolates with reduced susceptibility is not surprising, the rapid development of highly resistant organisms is of concern.
In summary, each fluoroquinolone was rapidly bactericidal in aerobic and anaerobic environments against staphylococci for which MICs were low. However, against isolates with reduced susceptibility, they may fail as monotherapy and could influence the genesis of organisms highly resistant to the entire antibiotic class.
Acknowledgments
D.H.W. and G.H.R. currently are affiliated with Ortho-McNeil Pharmaceutical. At the time of the study, they were employed by The University of Minnesota College of Pharmacy, Department of Experimental and Clinical Pharmacology.
This study was supported by a grant from Bayer Pharmaceuticals.
REFERENCES
- 1.Cooper, M. A., J. M. Andrews, and R. Wise. 1991. Bactericidal activity of sparfloxacin and ciprofloxacin under anaerobic conditions. J. Antimicrob. Chemother. 28:399-405. [DOI] [PubMed] [Google Scholar]
- 2.Hooper, D. C. 2000. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 31:S24-S28. [DOI] [PubMed] [Google Scholar]
- 3.Lewin, C. S., I. Morrissey, and J. T. Smith. 1991. The mode of action of quinolones: the paradox in activity of low and high concentrations and activity in the anaerobic environment. Eur. J. Clin. Microbiol. Infect. Dis. 10:240-248. [DOI] [PubMed] [Google Scholar]
- 4.Li, J., J. Turnidge, R. Milne, R. L. Nation, and K. Coulthard. 2001. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrissey, I., and J. T. Smith. 1994. The importance of oxygen in the killing of bacteria by ofloxacin and ciprofloxacin. Microbios 79:43-53. [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 7.O'Donnell, J. A., and S. P. Gelone. 2000. Antibacterial therapy. Fluoroquinolones. Infect. Dis. Clin. N. Am. 14:489-513. [DOI] [PubMed] [Google Scholar]
- 8.Wright, D. H., V. K. Herman, F. N. Konstantinides, and J. C. Rotschafer. 1998. Determination of quinolone antibiotics in growth media by reversed-phase high-performance liquid chromatography. J. Chromatogr. 709:97-104. [DOI] [PubMed] [Google Scholar]
- 9.Zabinski, R. A., A. J. Larsson, K. J. Walker, S. S. Gilliland, and J. C. Rotschafer. 1993. Elimination of quinolone antibiotic carryover through use of antibiotic-removal beads. Antimicrob. Agents Chemother. 37:1377-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zabinski, R. A., K. Vance-Bryan, A. J. Krinke, K. J. Walker, J. A. Moody, and J. C. Rotschafer. 1993. Evaluation of activity of temafloxacin against Bacteroides fragilis by an in vitro pharmacodynamic system. Antimicrob. Agents Chemother. 37:2454-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabinski, R. A., K. J. Walker, A. J. Larsson, J. A. Moody, G. W. Kaatz, and J. C. Rotschafer. 1995. Effect of aerobic and anaerobic environments on antistaphylococcal activities of five fluoroquinolones. Antimicrob. Agents Chemother. 39:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]