Abstract
The substituted benzimidazoles omeprazole, lansoprazole, rabeprazole, and pantoprazole were found to have in vitro activity against three different isolates of Plasmodium falciparum: D6 (which is chloroquine and pyrimethamine sensitive), W2 (chloroquine and pyrimethamine resistant), and TM91C235 (multidrug resistant). Lansoprazole and rabeprazole were the most effective against all three isolates, with a 50% inhibitory concentration (IC50) range of 7 to 11 μM. Omeprazole showed intermediate activity against D6 and W2 isolates, with IC50s of 27 to 28 μM, but had poor activity against TM91C235, with an IC50 of 76 μM. Pantoprazole was the least effective, with IC50s of 73 μM against D6, 53 μM against W2, and 39 μM against TM91C235. A pharmacophore model describing the important features responsible for potent activity of the drugs was developed using computational techniques of semiempirical quantum chemical methods and the three-dimensional QSAR procedure of the CATALYST software. The important features of the pharmacophore, according to the findings based on the CATALYST procedures, are the hydrogen bond acceptor and donor sites at the benzimidine nitrogen atoms and the two aromatic hydrophobic sites in the molecules. AM1 quantum chemical calculations identified the electrostatic potential surface surrounding the sulfoxide atom as crucial for potent activity.
Malaria is one of the most significant public health concerns in many tropical and subtropical regions of the world, with 40% of the world population exposed to malaria-causing parasites. Malaria Foundation International estimates that malaria causes up to 500 million clinical cases and over one million deaths each year; every 30 seconds, a child somewhere dies of malaria (Malaria Foundational International; http://www.malaria.org). It is rare to find a country in which malaria is endemic where chloroquine resistance has not been reported, and although the 4-aminoquinolone chloroquine is the drug most often reported to be associated with resistance, multidrug resistance is rapidly increasing in prevalence (4, 15, 21). New antimalarial drugs are urgently needed to overcome drug resistance.
Skinner-Adams et al. (17) tested the substituted benzimidazoles albendazole, thiabendazole, mebendazole, and omeprazole and two albendazole metabolites, albendazole sulfone and albendazole sulfoxide, against Plasmodium falciparum isolate 3D7. This group reported that omeprazole had rapid activity and a different stage-specific profile, with a 50% inhibitory concentration (IC50) range of 20 to 40 μM. On the basis of the findings of that study, we assayed four commercially available proton pump inhibitors (PPIs): omeprazole, lansoprazole, rabeprazole, and pantoprazole (Table 1). Our aim was to evaluate their efficacy in vitro against P. falciparum and attempt to elucidate a structure-activity relationship. This drug class is used in humans in combination with antibiotics to treat gastric infection with Helicobacter pylori and to reduce gastric acid output by inhibiting the H+- and K+-ATPase proton pumps. It is possible for these drugs to interfere with parasite nutrient transport through the same mechanism (7, 8, 19). The mechanism of action of these drugs against P. falciparum is unknown. The PPIs are acid labile. In humans, an active sulfenamide derivative is formed below a pH of 4, a condition which is found only in actively secreting gastric parietal cells. This intermediate allows a covalent disulfide bond to form with cysteine residues in the α-subunit of the gastric proton pump. It is unlikely that the pH is low enough anywhere in the malaria parasite to form the active intermediate. These drugs are all water soluble, and parenteral preparations of some are in clinical use.
TABLE 1.
Structures of the proton pump inhibitors
MATERIALS AND METHODS
Parasites and culture technique.
Three well-characterized P. falciparum clones with differing sensitivities to a wide range of antimalarial drugs were used for the antimalarial potency assays: the W2 clone, which was prepared from the Indochina I isolate; the D6 clone, from the African Sierra I/UNC isolate; and the TM91C235 isolate, from a patient in Thailand who failed mefloquine twice. The W2 clone is susceptible to mefloquine but resistant to chloroquine, sulfadoxine, pyrimethamine, and quinine; the D6 clone is naturally resistant to mefloquine but susceptible to chloroquine, sulfadoxine, pyrimethamine, and quinine; and the TM91C235 isolate is resistant to mefloquine, chloroquine, sulfadoxine, pyrimethamine, and quinine.
All isolates were maintained in the laboratory under previously described conditions (12, 14).
Test compounds.
Chloroquine and mefloquine were used as controls. Omeprazole was obtained from AstraZeneca, lansoprazole was obtained from Takeda Abbott Pharmaceuticals, rabeprazole was obtained from Eisai, and pantoprazole for intravenous administration was obtained through a United States Army pharmacy in Germany.
Parasite growth inhibition assay in vitro.
The in vitro assays were conducted using a modification of the semiautomated microdilution technique of Desjardins et al. (5) and Chulay et al. (3). Briefly, test compounds were dissolved in dimethyl sulfoxide and diluted 400-fold in RPMI 1640 culture medium supplemented with 25 mM NaHCO3 and 10% Albumax I (Gibco BRL, Grand Island, N.Y.). These solutions were subsequently serially diluted twofold using a Biomek 1000 robotic workstation (Beckman, Fullerton, Calif.) over 11 different concentrations from 50,000 ng/ml to 48.8 ng/ml. The parasites were exposed to serial dilutions of each compound for 24 h and incubated at 37°C with 5% CO2 and 90% N2 prior to the addition of [3H]hypoxanthine. After a further incubation of 18 h, parasite DNA was harvested from each microtiter well onto glass filters by using a Packard Filtermate 196 Harvester (Meriden, Conn.). The samples were washed to remove any unincorporated labeled hypoxanthine. Uptake of [3H]hypoxanthine was measured with a Packard topcount scintillation counter. Concentration-response data were analyzed by a nonlinear regression logistic dose response model, and the IC50 value for each compound was calculated (Table 2).
TABLE 2.
In vitro antimalarial activity against P. falciparum
| Drug | IC50 activity (μM) against:
|
||
|---|---|---|---|
| D6 | W2 | TM91C235 | |
| Rabeprazole | 9.8 | 10.0 | 7.0 |
| Lansoprazole | 9.3 | 7.5 | 11.2 |
| Omeprazole | 27.1 | 28.3 | 76.4 |
| Pantoprazole | 73.3 | 53.3 | 39.3 |
| Chloroquine | 0.008 | 0.10 | 0.15 |
Molecular modeling studies.
Molecular modeling studies were performed on the drugs, with computational techniques for calculating the stereoelectronic properties of electron surface density, electrostatic potentials, and charges used to develop a pharmacophore model. The stereoelectronic properties were calculated using the AM1 semiempirical quantum chemical methodology (6), and the pharmacophore modeling was performed using CATALYST software (version 4.6; Accelrys, San Diego, Calif.) on an SGI Octane workstation. We performed a detailed conformational search for each of the molecules by using the Monte Carlo search techniques (11) as implemented in SPARTAN software (version 4.0; Wavefunction, Inc., Irvine, Calif.) to find the minimum energy and highest abundance conformer. The geometry of this conformer was fully optimized by applying the AM1 quantum chemical procedure. We used CATALYST version 4.6 software (Accelrys) for generation of a pharmacophore for these drugs. The structures of the four drugs were edited in CATALYST and minimized to the closest local minimum using molecular mechanics (CHARMM force field) (2).
RESULTS AND DISCUSSION
Our study demonstrates that two of the substituted benzimidazoles used clinically as PPIs, rabeprazole and lansoprazole, had the best activity of this drug class against all three isolates tested in the concentration range of 7 to 11 μM. Omeprazole had an IC50 concentration range of 27 to 76 μM, and pantoprazole had an IC50 concentration range of 39 to 73 μM. PPIs are trapped by protonation in acidic environments when the pH is below their pKa of 4, allowing them to form disulfide bonds with a sulfur-containing amino acid. These drugs are, however, acid labile. The proton pumps of the food vacuole and acidocalcisome are potential targets for these PPIs when the pH is below 4 in these organelles. Saliba and Kirk published a paper discussing pH regulation via a V-type H+-ATPase in the plasma membrane of P. falciparum (16). This proton pump is an unlikely target for these drugs, since the cytoplasmic pH is not low enough to form the active derivative. Our findings support the published results for omeprazole (17) and show that rabeprazole and lansoprazole are effective against all of the P. falciparum clones tested at lower concentrations.
The ideal choice of conformers for structures being modeled should be the biologically relevant ones. No experimental structural data are available for the ligand bound protein, so the bioactive conformation of the ligand must be inferred from the optimized geometry and calculated steric and electrostatic properties that present the best complementarity for the assumed rigid binding site of the receptor protein. This assumption forms the basis of the pharmacophore concept for our model development (10). All stereoelectronic properties of the compounds were calculated based on this geometry.
The optimized geometric parameters of the drugs (Table 3) indicate that selected bond lengths and bond angles are virtually identical among the compounds, but some of the dihedral angles are remarkably different. Two observations of note are that (i) the S=O (sulfoxide) bond tends to remain in the same plane with the benzimidine ring, as evidenced from the near-180° orientation of the O1-S2-C6-N7 dihedral angle in all compounds, and (ii) the pyridine plane tends to remain closer to the sulfoxide bond in the more potent compounds, as seen from the consistent pattern of change in the O1-S2-C3-C4 (−88.1° to −96.3°), S2-C3-C4-C9 (172.1° to 178.5°), and S2-C3-C4-N5 (−7.86° to +2.3°) torsional angles. The orientation of the pyridine ring appears to influence activity, perhaps by enabling the electrons of the pyridine ring to interact more with the lone oxygen pairs of the sulfoxide bond in the more potent analogues.
TABLE 3.
Selected AM1 geometric parameters of the proton pump inhibitor drugs
| Drug | Bond distance (Å)
|
Bond angle (°)
|
Dihedral angle (°)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-H | O1…H | O1-S2-C6 | S2-C6-N8 | O1-S2-C3 | S2-C3-C4 | C3-C4-N5 | S2-C6-N7 | O1-S2-C6-N8 | O1-S2-C3-C4 | S2-C3-C4-C9 | S2-C3-C4-N5 | O1-S2-C6-N7 | |
| Rabeprazole | 0.98 | 2.585 | 103.2 | 123.5 | 105.2 | 111.0 | 117.7 | 124.2 | 3.8 | −88.9 | 172.1 | −7.86 | −179.8 |
| Lansoprazole | 0.98 | 2.590 | 103.3 | 123.5 | 105.3 | 111.2 | 117.8 | 124.1 | 5.7 | −88.1 | 172.8 | −7.2 | −178.1 |
| Omeprazole | 0.98 | 2.589 | 103.2 | 123.5 | 105.3 | 111.1 | 118.0 | 124.0 | 5.3 | −91.1 | 175.0 | −5.0 | −178.4 |
| Pantoprazole | 0.98 | 2.584 | 103.0 | 123.5 | 105.2 | 111.1 | 119.1 | 124.1 | 2.4 | −96.3 | −178.5 | 2.3 | 178.8 |
The calculated electronic properties (Table 4) and the electrostatic potential profiles of the drugs (Fig. 1; also see Fig. 2) show that the oxygen atom is the likely pharmacophoric site for the drugs. The sulfoxide oxygen atom has the most negative potential in all four molecules, which makes it the most nucleophilic site, and this oxygen atom (Fig. 1) maintains a fairly constant intrinsic nucleophilicity among the four drugs. The electronic environment in the region of this oxygen atom influences the activity against P. falciparum in vitro.
TABLE 4.
Selected stereoelectronic properties of the drugs as calculated by the AM1 method
| Drug | Charge (electrons)
|
MEP (kcal/mol)a
|
|||||
|---|---|---|---|---|---|---|---|
| N8 | N5 | C9 | O1 | S | Negative | Positive | |
| Rabeprazole | −0.11 | −0.56 | −0.37 | −0.62 | 0.84 | −96.6 | 34.1 |
| Lansoprazole | −0.08 | −0.56 | −0.44 | −0.62 | 0.86 | −92.7 | 37.5 |
| Omeprazole | −0.04 | −0.50 | −0.24 | −0.63 | 0.86 | −93.1 | 38.8 |
| Pantoprazole | −0.06 | −0.47 | −0.03 | −0.62 | 0.81 | −94.6 | 35.3 |
MEP, molecular electrostatic potential.
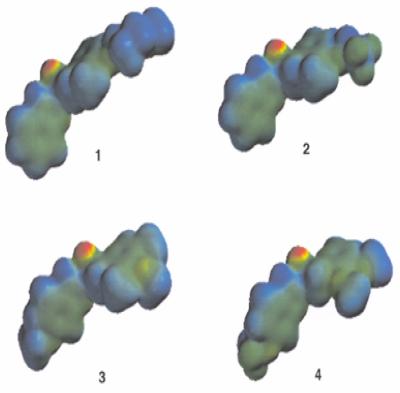
FIG. 1.
Molecular electrostatic potential isodensity of the molecular surfaces along with the optimized geometry, showing the sulfoxide oxygen atom as the site for most negative potential (deepest red region) or most nucleophilic site in the drugs. Panel 1, rabeprazole; panel 2, lansoprazole; panel 3, omeprazole; panel 4, pantoprazole. Electrostatic potential isodensity profiles: red, −97.000; red-yellow, −74.333; yellow-green, −51.667; green, −29.000; green-blue, −6.333; blue, 16.333; indigo, 38.000.
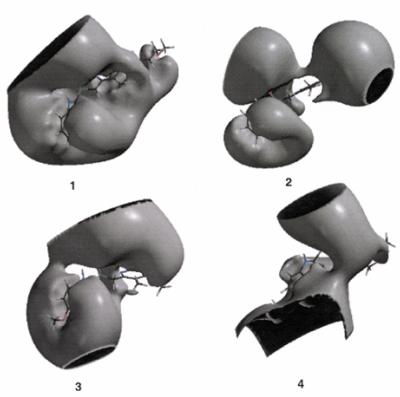
FIG. 2.
Three-dimensional electrostatic potential profiles of the compounds at −2.0 kcal/mol showing the regions covered by negative potentials and the hole-like or nucleophilic suction pump regions in the drugs. Panel 1, rabeprazole; panel 2, lansoprazole; panel 3, omeprazole; panel 4, pantoprazole.
The electrostatic potential maps beyond the van der Waals surface at a distance of 1.4 Å at −2.0 kcal/mol (Fig. 2) are consistent with the above pharmacophoric profiles of the drugs. The maps show lateral hole-like negative potential regions of various sizes in front of the sulfoxide oxygen atom in all the compounds, indicating that the sulfoxide oxygen atom is the primary focus for the recognition interaction. The recognition interactions are intermolecular forces underlying molecular recognition in all pharmacological and biological processes. These interactions arise primarily from electrostatic forces but may also include charge transfer interactions, ion-induced dipole interactions, induction forces, and dispersion forces that are more electrodynamic than electrostatic in nature in the molecule and are therefore the most likely pharmacophoric sites in the drugs (9). The electrostatic potential maps in the present study show two hole-like negative potential regions for the less potent compounds (Fig. 2). These regions may be viewed as “nucleophilic suction pumps” acting as powerful magnets toward the electrophilic sites in the receptor (1). These sites are likely to be recognized first from a distance by the receptor (13) and may be the driving force for the possible formation of a noncovalent Michaelis-type complex with the receptor (20). The characteristics of these profiles show that the antimalarial potency of the compounds decreases when two or more nucleophilic suction pumps are present on opposite sides of the molecule, with the decrease in potency probably arising from the conflicting driving forces for interaction with the electrophilic sites in the receptor. It is reasonable to conclude that the region near the sulfoxide oxygen atom is the pharmacophoric site of these drugs. Lansoprazole has two nucleophilic suction pumps but maintains potency similar to rabeprazole, probably by maintaining a relatively lower π electron density on the benzimidine ring, as is the case with rabeprazole.
Electrostatic charges of the molecules (Table 4) show a consistent pattern of change relative to potency in N5 (pyridinium) and N8 (benzidine) atoms. The charges in these atoms are less negative for the two less potent benzimidazoles. The charges associated with the C9 atom are the most negative for the more potent drugs. The charges associated with the O1 and S atoms remain unaltered in the compounds.
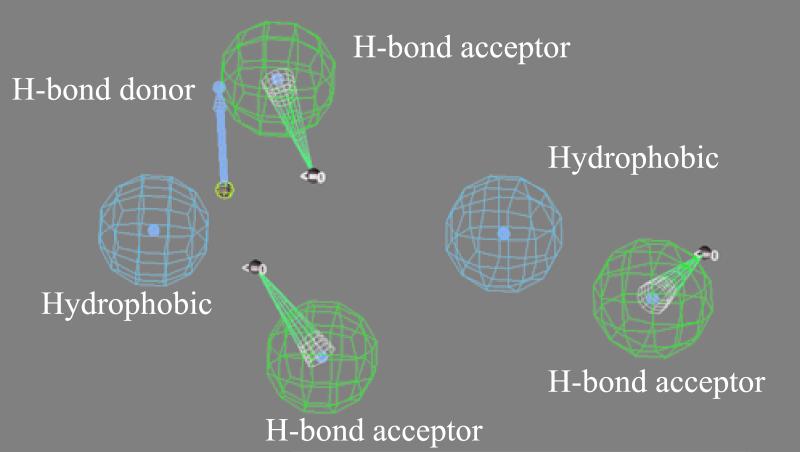
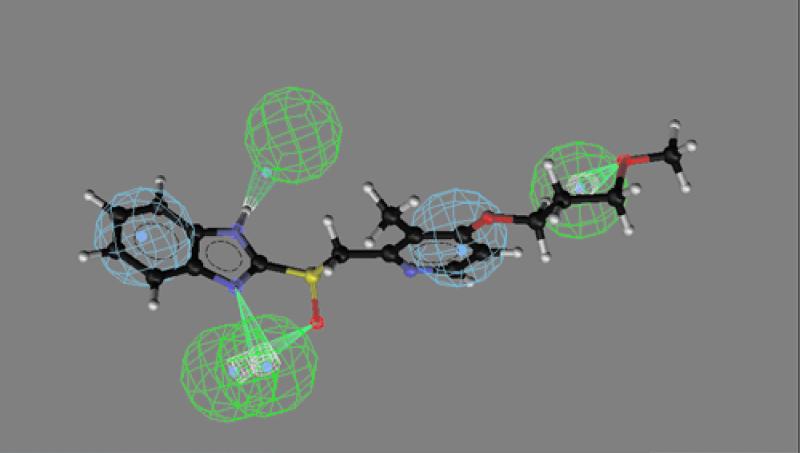
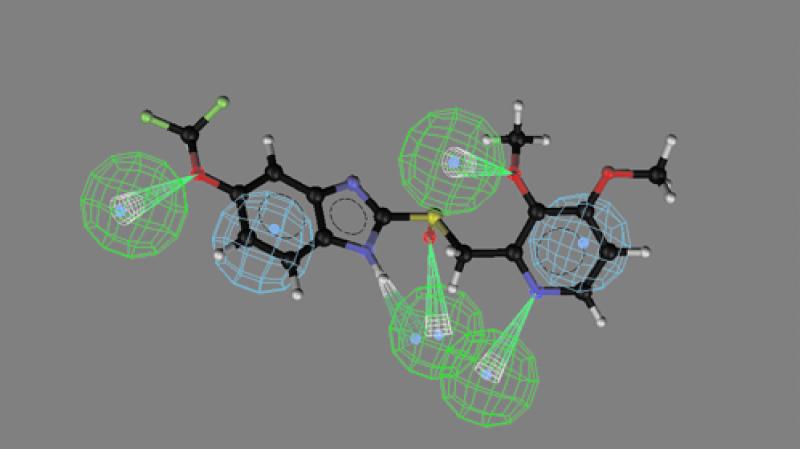
CATALYST software was used to identify the best three-dimensional arrangement of chemical functions to explain the variation in activity among the four drugs. The chemical functions used in the generation step of the pharmacophore included hydrogen bond acceptors, hydrogen bond donors, and hydrophobic interactions. By comparing the best-fit conformational match of the two more potent drugs, a pharmacophore model of the compound was produced containing 3 hydrogen bond acceptors, 1 hydrogen bond donor, and 2 aromatic hydrophobic sites (Fig. 3). The sulfoxide oxygen atom, the ether oxygen atom, and the benzimidine N7 atoms act as hydrogen bond acceptors, whereas the benzimidine secondary imino function acts as a hydrogen bond donor. The hydrophobic interaction areas are occupied by the two aromatic rings in the molecule. The mapping of this pharmacophore is shown for the more potent rabeprazole (Fig. 4) and the less potent pantoprazole (see Fig. 5). Inspection of these figures indicates that for potent activity, the drug should have both hydrogen bond acceptor and hydrogen bond donor sites as well as two aromatic hydrophobic sites. For pantoprazole, the hydrogen bond acceptor feature of the benzimidine N7 is totally absent in the mapping (Fig. 5). The presence and positions of the hydrogen bond acceptor and hydrogen bond donor sites are crucial for potent activity, and the geometry of the molecule is responsible for controlling the positioning of the chemical features. The conformational energy expended for the pharmacophore mapping of rabeprazole is 0.47 kcal/mol, but a similar mapping of pantoprazole has an energy cost of 4.7 kcal/mol.
FIG. 3.
Pharmacophoric features for antimalarial activity.
FIG. 4.
Mapping of the most potent analogue, rabeprazole, onto the pharmacophore model. All six features displayed in Fig. 3 are shown. The conformational energy expended for this mapping was 0.47 kcal/mol.
FIG. 5.
Mapping of the least potent analogue, pantoprazole, onto the pharmacophore model. The conformational energy expended for this mapping was 4.2 kcal/mol.
In conclusion, our molecular modeling study, which involves quantum chemical calculations and CATALYST-based three-dimensional pharmacophore generation, demonstrates that the orientation of the electronic environment associated with the benzimidine and pyridinium rings in these drugs is crucial for potent activity. We used this pharmacophore model as a template to search for new compounds in our chemical information system database of approximately 250,000 compounds (Chemical Information System; Walter Reed Army Institute of Research, Silver Spring, Md.). We identified 128 compounds with similar features. Three of these compounds have documented antimalarial properties in our in vivo mouse screen.
Further studies with congeners of rabeprazole and lansoprazole that satisfy this model are warranted, the results of which will allow us to refine the pharmacophore. In vivo testing in mice will be conducted.
Tina Skinner-Adams recently evaluated drug combinations in vitro and found that omeprazole and quinine are synergistic but that omeprazole and chloroquine are antagonistic (18). Doxycycline and azithromycin are antibiotics with known activity against P. falciparum. The present eradication regimen for gastric infection by Helicobacter pylori is a combination of a PPI with two antibiotics, most commonly amoxicillin and clarithromycin. It is possible that a combination of doxycycline or azithromycin with a PPI would prove synergistic against malaria.
Acknowledgments
We thank AstraZeneca, Takeda Abbott Pharmaceuticals, and Eisai for their donation of drug powder for the study.
We also thank Lucia Gerena for her technical assistance with the in vitro assays.
REFERENCES
- 1.Bhattacharjee, A. K., and J. M. Karle. 1996. Molecular electronic properties of a series of 4-quinolinecarbinolamines define antimalarial activity profile. J. Med. Chem. 39:4622-4629. [DOI] [PubMed] [Google Scholar]
- 2.Brooks, B. R., R. E. Bruccoleri, B. D. Olafson, D. J. States, S. Swaminathan, and M. Karplus. 1983. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Computat. Chem. 20:187-217. [Google Scholar]
- 3.Chulay, J. D., J. D. Haynes, and C. L. Diggs. 1983. Plasmodium falciparum: assessment of in vitro growth by [3H]hypoxanthine incorporation. Exp. Parisitol. 55:138-146. [DOI] [PubMed] [Google Scholar]
- 4.Cowman, A. F., and S. J. Foote. 1990. Chemotherapy and drug resistance in malaria. Int. J. Parasitol. 20:503-513. [DOI] [PubMed] [Google Scholar]
- 5.Desjardins, R. E., C. J. Canfield, D. E. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewar, M. J. S., E. G. Zoebisch, E. F. Horsley, and J. J. P. Stewart. 1985. New general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 107:3902-3909. [Google Scholar]
- 7.Karcz, S. R., V. R. Hermann, and A. F. Cowman. 1994. Cloning and characterization of the vacuolar ATPase B subunit from Plasmodium falciparum. Mol. Biochem. Parasitol. 65:123-133. [DOI] [PubMed] [Google Scholar]
- 8.Karcz, S. R., V. R. Herrmann, and A. F. Cowman. 1993. Cloning and characterization of a vacuolar ATPase A subunit homologue from Plasmodium falciparum. Mol. Biochem. Parasitol. 58:333-344. [DOI] [PubMed] [Google Scholar]
- 9.Lipkowitz, K. B., and D. B. Boyd. 1997. Reviews in computational chemistry, vol. 11, p. 243-247. Wiley-VCH, Weinheim, Germany.
- 10.Marshall, G. R., and R. D. Cramer III. 1988. Three dimensional structure-activity relationships. Trends Pharmacol. Sci. 9:285-289. [DOI] [PubMed] [Google Scholar]
- 11.Metropolis, N., A. Rosenbluth, M. Rosenbluth, A. Tellor, and E. Teller. 1953. Equation of state calculations by fast computing machines. J. Chem. Phys. 21:1087-1092. [Google Scholar]
- 12.Milhous, W. K., N. J. Weatherly, J. H. Bowdre, and R. E. Desjardins. 1985. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob. Agents Chemother. 27:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray, J. S., B. A. Zilles, K. Jayaswuya, and P. Politzer. 1986. Comparative analysis of the electro-static potentials of dibenzofuran and some dibenzo-p-dioxins. J. Am. Chem. Soc. 108:915-918. [Google Scholar]
- 14.Oduola, A. M. J., N. J. Weatherly, J. H. Bowdre, and R. E. Desjardins. 1988. Plasmodium falciparum—cloning by single erythrocyte manipulation and heterogeneity in vitro. Exp. Parasitol. 66:86-95. [DOI] [PubMed] [Google Scholar]
- 15.Peters, W. 1990. Drug resistance in malaria. Recenti Prog. Med. 81:749-753. [PubMed] [Google Scholar]
- 16.Saliba, K. J., and K. Kirk. 1999. pH regulation in the intracellular malaria parasite, Plasmodium falciparum. H(+) extrusion via a v-type h(+)-atpase. J. Biol. Chem. 274:33213-33219. [DOI] [PubMed] [Google Scholar]
- 17.Skinner-Adams, T. S., T. M. E. Davis, L. S. Manning, and W. A. Johnston. 1997. The efficacy of benzimidazole drugs against Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 91:580-584. [DOI] [PubMed] [Google Scholar]
- 18.Skinner-Adams, T., and T. M. Davis. 1999. Synergistic in vitro antimalarial activity of omeprazole and quinine. Antimicrob. Agents Chemother. 43:1304-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanabe, K. 1990. Iron metabolism in malaria-infected erythrocytes. Blood Cells 16:437-449. [PubMed] [Google Scholar]
- 20.Voet, D., and J. G. Voet. 1995. Biochemistry, 2nd ed., p. 351. John Wiley & Sons, New York, N.Y.
- 21.White, N. J. 1992. Antimalarial drug resistance: the pace quickens. J. Antimicrob. Chemother. 30:571-585. [DOI] [PubMed] [Google Scholar]