Abstract
Several steps in the pathogenesis of a Plasmodium falciparum infection depend on interactions of parasite surface proteins with negatively charged sugars on the surface of host cells such as sialate residues or glycosaminoglycans. For these reasons, our previous studies examining agents that interfere with heparan sulfate-protein binding during amyloidogenesis suggested that short-chain aliphatic polysulfonates may prove useful as antimalarial agents. A series of related polysulfonates were synthesized and assessed both in tissue culture with the asexual stages of P. falciparum in human red blood cells and in vivo by use of Plasmodium berghei infections in mice. Poly(vinylsulfonate sodium salt) (molecular weight range, 1,500 to 3,000) proved effective in interfering with P. falciparum merozoite entry into human red blood cells and significantly delaying the increase in the level of P. berghei parasitemia in mice. The concept that anionic molecules that mimic large polysaccharide structures may have antimalarial properties has been suggested and examined previously. Our results suggest that related anionic agents [poly(vinylsulfonate sodium salt)-like molecules] orders of magnitude smaller than those previously considered may prove useful in abrogating merozoite entry into erythrocytes and may potentially block sporozoite entry into liver cells. Structure-activity studies conducted to enhance these properties may provide compounds with scope for significant further analysis and development.
Chloroquine-resistant Plasmodium falciparum malaria was first recognized over 40 years ago and has since spread to almost all areas where malaria is endemic (30). When chloroquine-resistant malaria extended into the areas of Africa with high rates of malaria parasite transmission, it raised fears of an impending public health crisis since switching to alternative antimalarials such as mefloquine, artemisinin derivatives, halofantrine, or quinine is unaffordable for many countries in sub-Saharan Africa (41). Recent reports indicate that the rates of mortality due to a widespread resurgence of malaria are now escalating (21). To meet this challenge, novel and inexpensive antimalarials must be found. The asexual schizogonic blood stage is the most frequently targeted point in the parasite's life cycle (10). Symptoms of the disease begin to occur during this stage; and the consequences of the parasite's presence can include anemia, organ dysfunction, coma, and even death (3).
The majority of the antimalarials available at present (e.g., quinine, mefloquine, quinidine, and chloroquine) are quinolines that function by inhibiting the polymerization and detoxification of heme into an insoluble pigment (hemazoin) (11). This has led to problems with cross-resistance between related antimalarials and limits the time during which the parasite is vulnerable to treatment to the second half of its intraerythrocytic life cycle. Other classes of antimalarials exist, such as antifolates, which target the parasite's bifunctional dihydrofolate reductase-thymidylate synthase enzyme (17), and atovaquone, which inhibits the parasite's mitochondrial electron transport and mitochondrial membrane potential. However, inhibitors of other phases of the asexual life cycle would be useful. Another potential target in the development of antimalarials is the merozoite invasion process (6).
The invasion process involves a release of merozoites from a parasitized erythrocyte (RBC), followed by an initial attachment and reorientation event, in part dependent on an ionic interaction between components on the merozoite and those on the RBC (7). Irreversible attachment and junction formation ensue, followed by formation of the parasitophorous vacuole and insertion of the merozoite into the RBC. This process relies on specific ligands and receptors; however, the identities of these combinations are still being determined (6). To date, no commercially available antimalarial agents that specifically inhibit this step in the life cycle have been identified. Nevertheless, there have been several reports that saccharide anions inhibit both the invasion of RBCs by merozoites (8) and the invasion of hepatic cells by sporozoites (28, 29). Furthermore, heparin, dextran sulfate, fucoidin, and chondroitin sulfate all inhibit one or several parasite-host interactions; however, their large polydisperse nature and other in vivo activities have hampered their development as therapeutic agents (35).
We have noted that several stages of malaria parasite infection involve binding interactions between proteins on the surface of the parasite and negatively charged sugars on the surface of host cells, such as sialate residues or sulfated glycosaminoglycans (GAGs). Examples include sporozoite binding to hepatocyte heparan sulfate (42) and infected RBC binding to endothelial chondroitin sulfate (14, 16, 23, 34) and placental chondroitin sulfate (1, 15, 22, 33). Several of us (R.K., W.A.S., and S.B.) have had extensive experience exploring negatively charged sugar polymer (GAG)-protein interactions related to amyloidogenesis (4, 19, 27). In the latter case, the GAG-protein binding rapidly changes the conformation of the amyloidogenic protein, leading to protofilaments (19, 24) and amyloid fibrils which adversely affect adjacent parenchymal cells (e.g., in patients with Alzheimer's disease). Agents that prevent this GAG-protein interaction have proved of value in inhibiting both the deposition of amyloid and the mobilization of preexisting deposits (19). One such class of compounds is short-chain aliphatic anionic sulfonates.
As part of a program extending our GAG-protein binding studies to the anionic sugar polymer-cationic malarial protein binding issue, we have been examining structural details of GAG binding to sporozoite and merozoite surface proteins. We have determined that aliphatic polysulfonates have antimalarial properties both in vitro and in vivo at concentrations that may lead to potentially useful therapeutic agents. While the exact mechanism of their action is not yet known, they appear to be potent inhibitors of the process whereby the merozoite invades the host RBC, and they may therefore constitute a novel class of antimalarials.
MATERIALS AND METHODS
Chemical synthesis. (i) Poly(vinylsulfonate sodium salt) I (compound 1).
A supply of poly(vinylsulfonate sodium salt) was obtained from Aldrich Chemical Company (catalog no. 27,842-4) as a 25% (wt/wt) solution in water, and the solution was processed as follows to provide a sample of compound 1. The solution (2 liters) was concentrated under reduced pressure to half of its volume. Ethanol (200 ml) was added, followed by the addition of activated carbon (50 g). The mixture was warmed on a steam bath for 20 min and then filtered through Celite, and the solvent was removed under reduced pressure to give a light-yellowish oil. The oil was dried in a vacuum oven (50°C) for 3 days to give an amorphous solid (486 g). The molecular weight distribution was determined by gel permeation chromatography by the American Polymer Standards Corporation, as follows: number-average molecular weight (Mn) = 1,800; weight-average molecular weight (Mw) = 3,050; z-average molecular weight (Mz) = 5,400; Mw/Mn (polydispersity index) = 1.69.
(ii) Poly(vinylsulfonate sodium salt) II (compound 2).
Poly(vinylsulfonate sodium salt) II was prepared by a radical polymerization of sodium vinylsulfonate in the presence of sodium persulfate. Each of the solutions, sodium vinylsulfonate (catalog no. 27,841-6; Aldrich) as 160 ml of a 25% (wt/wt) solution in water (160 ml) and sodium persulfate (1.6 g) in H2O (30 ml), was purged with argon for 30 min, and then the solutions were mixed. The mixture was heated (under argon) at 80°C in an oil bath for 17 h. The reaction mixture was concentrated under reduced pressure to 100 ml; addition of methanol (1 liter) gave a white solid which was dried in a vacuum oven (75°C) overnight to give a sample of compound 2 (45 g). The molecular weight distribution was determined by gel permeation chromatography by the American Polymer Standards Corporation, as follows: Mn = 1,600; Mw = 2,000; Mz = 2,700; Mw/Mn = 1.25.
(iii) Sodium 1,2-ethanedisulfonate (compound 3), sodium 1,3-propanedisulfonate (compound 4), sodium 1,4-butanedisulfonate (compound 5), sodium 1,5-pentanedisulfonate (compound 6), sodium 1,6-hexanedisulfonate (compound 7), and sodium 1,9-nonanedisulfonate (compound 8).
The disulfonates (compounds 3 to 8) were prepared as their sodium salts by way of the reaction of sodium sulfite with the corresponding dibromide (see the report by Stone [39]). In each case, the 1H and 13C nuclear magnetic resonance spectra and the electron-spray ionization-mass spectrometry spectrum were consistent with the assigned structure.
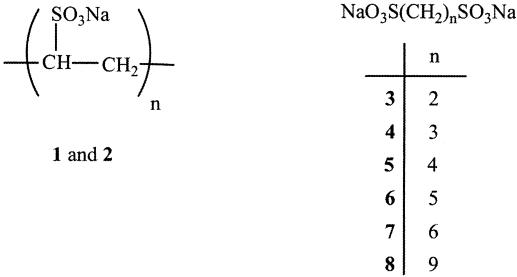
The structures of the candidate compounds (compounds 1 to 8) are shown in Fig. 1.
FIG. 1.
Structures of candidate compounds 1 to 8. The boldface numbers represent the compound numbers
Parasite lines and culture.
P. falciparum cultures were grown in human type A-positive blood obtained by venipuncture of volunteers. Cultures of the laboratory line ItG were maintained by the method of Trager and Jensen (40) with RPMI 1640 supplemented with 10% human serum and 50 μM hypoxanthine (RPMI-A). In vitro P. falciparum susceptibility testing was performed by a lactate dehydrogenase (LDH) enzyme assay specific for the LDH found in Plasmodium (pLDH) (20, 31). Briefly, the compounds to be tested were dissolved in RPMI-A, and the solutions were filtered by passing them through a 0.22-μm-pore-size polycarbonate filter and then serially diluted in duplicate in a 96-well plate to produce a compound gradient with twofold dilutions. Equal amounts of parasite culture (5% hematocrit, 5% parasitemia) were added to each well, and the plates were then incubated at 37°C in 95% N2-3% CO2-2%O2 for 72 h. The contents of the wells were then resuspended, and a 10-μl sample was removed and added to 100 μl of the pLDH enzyme assay mixture. After 1 h the absorbance of the wells at 650 nm was determined with a microplate reader. The 50% inhibitory concentrations (IC50s) of individual compounds were determined by a nonlinear regression analysis of the dose-response curve. The initial concentrations used in vitro were determined by serially diluting 1-mg/ml solutions of the candidate compounds.
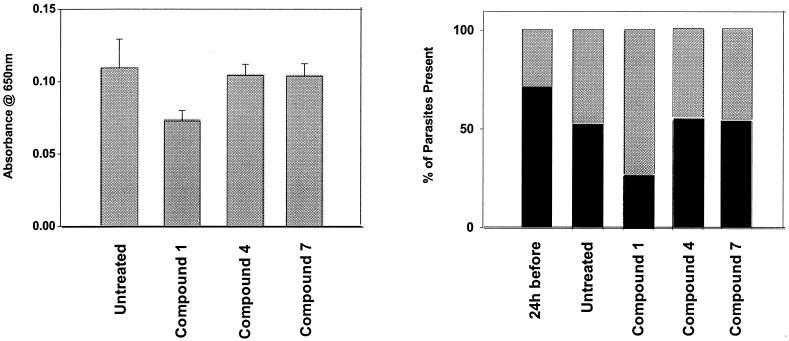
In some experiments asynchronous parasite cultures (1 ml) were either untreated (controls) or treated with 500 μg of compound 1, 4, or 7 per ml for 24 h. The RBCs were then washed once in RPMI-A prior to the addition of 10-μl aliquots to the plates for the LDH assay and were used to produce blood films that were stained with Giemsa. The percentage of parasites present as ring or mature stages were then determined for each sample.
Determination of compound activity in mice.
Female BALB/c mice (age, 6 to 8 weeks; Charles Rivers, Montreal, Quebec, Canada) were used in the study, and for each experiment the mice were divided into four groups of eight mice each. Each group was infected with Plasmodium berghei as described below. Twenty-four hours after infection the compound to be tested was administered by intraperitoneal (i.p.) injection every 12 h (9 a.m. and 9 p.m.) for at least 10 days. The quantities administered in vivo were based on the effective concentrations determined in culture, the assumption that the compound was distributed evenly within the animal, and a mouse weight of 25 g (i.e., an animal volume of 25 ml). In some experiments a previously demonstrated effective concentration either was administered 24 h before parasite inoculation and treatment was continued every 12 h (9 a.m. and 9 p.m.) for at least 10 days or was administered 24 h after parasite inoculation and treatment was discontinued after 7 days. Animals were killed at the termination of the experiment by CO2 narcosis.
The compounds were dissolved in distilled water and injected i.p. in a volume of 100 μl. Sufficient compound was prepared in water for entire experiments, and 5-ml aliquots were stored frozen at −20°C until they were used. Aliquots were thawed slowly at 4°C before use. Unfrozen compound was always kept at 4°C, but at no time did the compound remain unfrozen for more than 48 h.
The P. berghei organisms used for the i.p injections were obtained from the blood of previously infected untreated mice that had been infected with the organism for 10 to 14 days. Their parasite loads were of the order of 40 to 50 intracellular organisms/100 RBCs. Such mice were exsanguinated by cardiac puncture after CO2 narcosis, and the blood was immediately mixed with Alsever's solution (9.5 ml of glycerol, 2.33 g of glucose, 0.52 g of NaCl, and 1.0 g of sodium citrate made to 100 ml with distilled water) at a volume ratio of 1:3 (blood:solution). It was then divided into 0.5-ml aliquots and frozen at −70°C until it was required. When they were required, aliquots of diluted blood were thawed and mixed, and 100 μl was injected i.p. into each mouse. In most experiments a single injection was sufficient to raise infection levels to over 40 intracellular parasites/100 RBCs in untreated animals in 10 to 11 days. When the aliquots were stored for longer periods, the postinoculation increase in parasite load was slower than the loads obtained when fresher aliquots were used.
To monitor the progression of the parasitemia in each mouse, 10 to 15 μl of blood was collected from the tail tip once every 24 h and placed into a heparinized glass capillary tube; and a blood smear was prepared, air dried, fixed in a 100% methanol bath for 2 min, immediately stained with Giemsa for 30 min, and rinsed in water. The slides were then air dried and dipped in Hemo-D solvent (a clearing agent; Fisher), and a coverslip was placed with Permount.
The number of intracellular parasites per 100 RBCs was determined by image analysis. The imaging system consisted of a Leitz Diaplan microscope with a ×40 objective and a ×10 eyepiece. A Hitachi model HV-C10U CCD television camera was mounted on the microscope, and the image was transferred to the screen of a computer with a Pentium-type processor and digitized. The individual RBC images on the screen were sharp and considerably larger than those seen under a microscopic high-power field at a ×1,000 magnification. The parasites in infected cells were easily recognized. The morphological parameters describing an average RBC were determined experimentally and entered into the program to be used with each field examined. When tested and validated against manual counts, in regions where the cells were well dispersed, the program determined the number of RBCs per field to a level with an error of 1 to 2%. The intraerythrocytic parasites were easily recognized, and the numbers were counted manually from the screen of the computer monitor. The total numbers of RBCs per field were determined by image analysis. A minimum of 500 cells were counted for each slide. The result was expressed as the numbers of intracellular parasites/100 RBCs. The image analysis program (MC-5, version 4.0, beta 2.0) is from MCID Imaging Research Inc., Brock University, St. Catharines, Ontario, Canada L2S 3A1.
RESULTS
Effects of compounds on P. falciparum in culture.
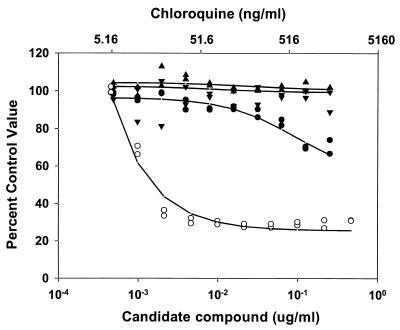
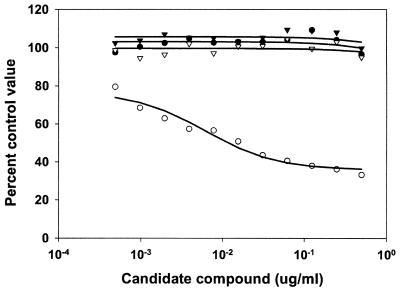
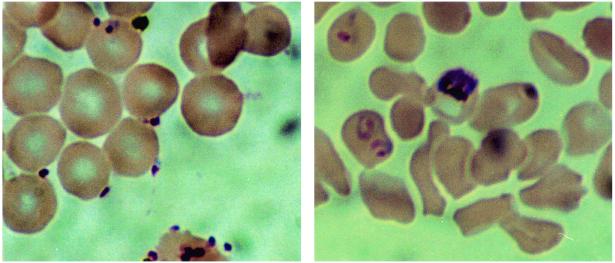
An initial evaluation of the compounds was undertaken to determine if the compounds had antimalarial properties in vitro. When the compounds were used in a 48-h antimalarial activity assay that measured compound concentration versus pLDH activity in asynchronous cultures, it was observed that compounds 4 and 7 had no detectable effect on the outcome of the assay. Similar experiments indicated that compound 1 had a detectable effect (Fig. 2). Microscopic examination of the parasites in the wells with the highest concentration of compound 1 indicated that there were an unusual number of free merozoites. Merozoites that had successfully invaded RBCs appeared to have developed normally. When the assay period was extended to 72 h and the parasite cultures were synchronized to the ring stage of development by sorbitol lysis, a more dramatic effect of compound 1 on the cultures was observed (Fig. 3). From this assay it was determined that compounds 4 and 7 had IC50s of >20 mM and that compound 1 had an IC50 of 0.2 μM on the basis of a molecular weight of 3,050 (Table 1). Examination of synchronized cultures with compounds 4 and 1 that were allowed to mature to the point of merozoite release suggested that merozoites failed to adhere to human RBCs in the presence of compound 1 (Fig. 4). Among a series of compounds related to compound 1, compound 2 had an IC50 of 6 ± 2 μM on the basis of a molecular weight of 2,000 (Table 1).
FIG. 2.
Effects of chloroquine and compounds 1, 4, and 7 on the viabilities of nonsynchronized P. falciparum merozoites in tissue culture. P. falciparum cultures were exposed to various concentrations of either a candidate compound (lower scale) or chloroquine (upper scale) for 48 h before the degree of parasite viability was determined by an LDH assay (see Materials and Methods). The percentage of the control activity was determined by comparing the values obtained in treated wells to those obtained in untreated wells. The datum points indicate the results obtained with compounds 1 (•), 4 (▴), and 7 (▾) and chloroquine (○), with lines of best fit shown.
FIG. 3.
Effects of candidate compounds on the viabilities of sorbitol-lysed synchronized P. falciparum merozoites in tissue culture. P. falciparum cultures were exposed to various concentrations of a candidate compound for 48 h before the degree of parasite viability was determined by an LDH assay (see Materials and Methods). The percentage of the control activity was determined by comparing the values obtained in treated wells to those obtained in untreated wells. The datum points indicate the results obtained with compound 4 (•), compound 7 (▾), no treatment (▿), and compound 1 (○), with lines of best fit shown. Compounds 4 and 7 had IC50s greater than 1 mg/ml. The IC50 of compound 1 was 0.2 μM.
TABLE 1.
IC50s of a series of candidate compounds for P. falciparum viability in human RBC cultures
| Compound | IC50 |
|---|---|
| 1 | 0.2 μM |
| 2 | 6 ± 2 μM |
| 3 | >20 mM |
| 4 | >20 mM |
| 5 | >20 mM |
| 6 | 12 ± 1 mM |
| 7 | >20 mM |
| 8 | ≥20 mM |
FIG. 4.
Effects of candidate compounds on invasion of merozoites. P. falciparum cultures were grown in the presence of 6 μg of compound 1 per ml (left panel) or 6 μg of compound 4 per ml (right panel). After 24 h, samples of the cultures were removed and thin films were produced, fixed with methanol, and stained with 3% Giemsa stain in water for 30 min. The panels indicate representative fields of cultured RBCs-malarial parasites observed by microscopy. Note the extracellular locations of the parasites in the left panel. Magnifications, ×1,000.
Assessment of the proportions of ring and mature stages of the parasites after 24 h of treatment with compound 1, 4, or 7 revealed that the last two compounds had little effect on the different stages compared to the effect of no treatment. In contrast, compound 1 had an effect on the viability of the parasites and caused a significant reduction in the proportion of ring forms (Fig. 5).
FIG. 5.
Asynchronous parasite cultures (1 ml) were either untreated or treated with 500 μg of compound 1, 4, or 7 per ml for 24 h. The RBCs were then washed once in RPMI-A prior to addition of 10-μl aliquots to plates for the LDH assay (the values are the averages ± standard deviations of four values) and were used to produce blood films that were stained with Giemsa. The percentages of parasites present as ring or mature stages were then determined for each sample (n = 200 cells in each case).
In vivo observations with P. berghei.
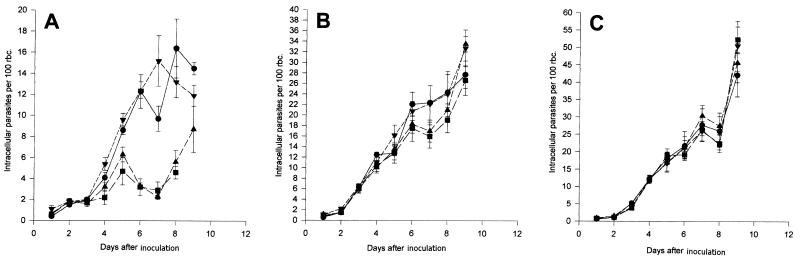
Figure 6A illustrates the effects of different doses of compound 1 on the progression of the parasitemia in vivo. Of significant interest was the effect of compound 1 at doses of 4.8 and 38.4 mg twice daily. There was considerable inhibition of the parasitemia over the first 7 days in the treated animals compared to that in the untreated animals and those receiving a dose of 0.6 mg twice daily. After 7 days the parasites appeared to break free of the inhibitory effects of this compound, with parasite levels increasing steadily. A two-way analysis of variance (ANOVA) examining between-group variations indicated a highly significant inhibition (P < 0.001) by the two higher doses of compound 1.
FIG. 6.
Effects of compounds 1 (A), 4 (B), and 7 (C) on the time course of P. berghei parasitemia expressed as numbers of intracellular parasites/100 RBCs. Parasites were inoculated i.p., and treatment with compound 1, 4, or 7 was started 24 h later. Each dose of agent was given i.p. at 12-h intervals for a period of 9 days. •, no treatment; ▾, treatment with a dose of 0.6 mg; ▴, treatment with a dose of 4.8 mg; ▪, treatment with a dose of 38.4 mg. (A) Each point is the mean ± standard error of the mean for eight animals per time point. An ANOVA of between-group comparisons revealed significant differences (P < 0.001). (B) Each point is the mean ± standard error of the mean for eight animals per time point. An ANOVA of between-group comparisons revealed significant differences (P < 0.012). (C) Each point is the mean ± standard error of the mean for eight animals per time point. An ANOVA of between-group comparisons revealed no significant differences.
In contrast to the effects of compound 1, compound 4 (Fig. 6B), the monomeric unit of compound 1, at the two higher doses had a transient lesser inhibitory effect, but an inhibitory effect that was nonetheless significant (P < 0.012), between days 5 and 9. Compound 7, an additional compound related to compound 1 in which the charged groups are spaced farther apart, had relatively little effect on progression of the infection (Fig. 6C). Such a compound serves as a good negative control.
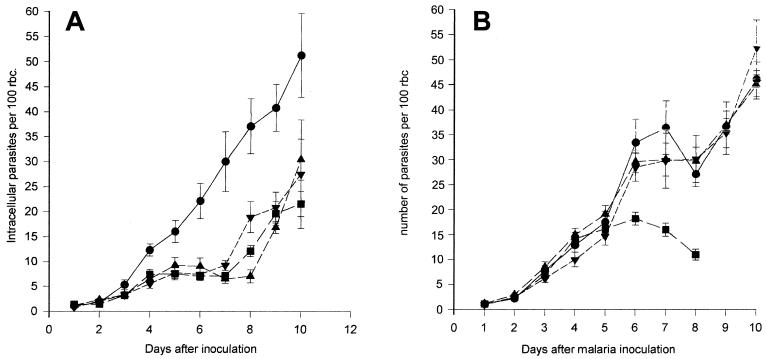
Figure 7A illustrates the effect of compound 1 administered at 4.8 mg twice daily beginning either 24 h before or 24 h after parasite inoculation. This compound also considerably inhibited the parasitemia over the first 7 days after inoculation compared to the level of parasitemia in untreated animals (P < 0.001), but it was not more effective than treatment postinoculation. Figure 7B illustrates the effects of different doses of compound 2.
FIG. 7.
(A) Effects of poly(vinylsulfonate sodium salt) I (compound 1; 4.8 mg/dose) administered either before or after parasite inoculation on the time course of P. berghei parasitemia, expressed as the numbers of intracellular parasites/100 RBCs. Parasites were inoculated i.p., and treatment with compound 1 was started either 24 h before or 24 h after inoculation. Compound 1 was given i.p. at 12-h intervals for a period of 10 days. •, no treatment; ▪, treatment with compound 1 starting 24 h after inoculation; ▾, treatment with compound 1 starting 24 h after inoculation and ending after day 7; ▴, treatment with compound 1 starting 24 h before inoculation. Each point is the mean ± standard error of the mean for eight animals per time point. An ANOVA of between-group comparisons revealed significant differences (P < 0.001). (B) Effects of different doses of poly(vinylsulfonate sodium salt) II (compound 2) on the time course of P. berghei parasitemia, expressed as the numbers of intracellular parasites/100 RBCs. Parasites were inoculated i.p., and treatment with compound 2 was started 24 h later. Each dose of compound 2 was given i.p. at 12-h intervals for a period of 9 days. •, no treatment; ▾, treatment with a dose of 125 μg; ▴, treatment with a dose of 500 μg; ▪, treatment with a dose of 2 mg. The highest dose proved to have an adverse effect on animal well-being, and these animals were euthanized after 8 days. Each point is the mean ± standard error of the mean for eight animals per time point. An ANOVA of between-group comparisons revealed significant differences (P < 0.001).
The infection dynamics appear to be slightly different in each of the in vivo experiments, with a more gradual increase in the level of parasitemia in some experiments compared with the rate of increase in others. This is likely due to the viabilities of the parasites in aliquots of infected mouse blood that are stored for different periods of times and that are used to inoculate the mice.
As early as the second day of treatment compounds 1 and 2 had adverse effects on the health of the mice. Although the parasite levels were significantly lower in mice treated with compounds 1 and 2 than in the untreated control mice, animals treated with compounds 1 and 2 were much less active than the animals in the untreated group, their fur was ruffled, and at the time they were killed their tissues appeared to be dehydrated. Gross inspection of the organs of such animals failed to reveal any obvious pathological features such as differences in size, color, or shape. No such adverse effects could be demonstrated when compounds 1 and 2 were given orally in antiamyloid studies (19) rather than by the i.p. route used in the present studies. The route of administration of compounds 1 and 2 plays a role in the toxic manifestations. The antimalarial properties of compounds 1 and 2 were likely not dependent on their in vivo toxic effects, as the antimalarial effect was also seen in vitro. Furthermore, compound 7 exhibited toxicity but was not effective in vivo.
DISCUSSION
The compounds studied were originally designed to explore their inhibitory properties on heparan sulfate-amyloid protein binding. This heparan sulfate-protein interaction rapidly alters the conformation of amyloidogenic proteins such as serum amyloid A, the precursor to inflammation-associated amyloid (24), and Aβ, the peptide in Alzheimer's disease-associated amyloid (12, 19, 25, 26), conferring upon them significant increases in β-sheet structure, the characteristic protein conformation of amyloids. Our initial attempts to find heparan sulfate-protein binding inhibitors focused on sulfonated and sulfated disaccharides as analogues of the heparan sulfate structure, and they proved to have the required activities (unpublished data). Unfortunately, they also had significant anticoagulant properties. However, the acyclic compounds retained the antiamyloid properties but did not display the unwanted anticoagulant effects (19).
In several ways many of the steps in the malarial parasite invasion process are analogous to the GAG-amyloid protein interaction seen in amyloidogenesis. There is robust evidence indicating that the P. falciparum sporozoite interacts with the host's hepatocyte through a heparan sulfate proteoglycan on the liver cell surface and its own circumsporozoite protein during its entry into the liver cell (37, 42). Native remnant lipoprotein particles which use the same binding process with hepatocytes compete effectively with the sporozoites for entry into liver cells (13, 38). P. falciparum RBC membrane protein 1 (PfEMP-1) is expressed on the surfaces of parasitized RBCs. It possesses heparan sulfate-like binding properties that may be important in the formation of RBC rosettes (5). Furthermore, infected RBCs expressing PfEMP-1 on their surfaces can adhere to the chondroitin sulfate present on endothelial thrombomodulin (32, 34), can potentially adhere to endothelial cell surface heparan sulfate (9), and can adhere to the chondroitin sulfate in the placenta during pregnancy (1, 15, 22, 33). Moreover, merozoite entry into RBCs may also depend, in part, on ionic interactions (7). The success that we had interfering with amyloidogenesis by using agents with low molecular weights that blocked GAG-protein interactions suggested that the same strategy might be useful in interfering with some of these steps during the malarial parasite invasion process. Such a concept has, in fact, been considered in the past (8, 28, 29), but the previous approaches used high-molecular-weight compounds (compounds with molecular weights of the order of 500,000) very similar in structure to the GAGs themselves (8). In this context our present findings indicate that molecules orders of magnitude smaller than those previously considered as antimalarials have many desirable properties and should be considered lead compounds for additional exploration and optimization. Such studies are ongoing with agents structurally related to poly(vinylsulfonate), with promising results (unpublished data). The poly(vinylsulfonate) appears to exert its effect by inhibiting merozoite entry into RBCs, a step which may involve an ionic heparan sulfate-like binding interaction (5). Precisely which merozoite surface-RBC surface molecular interaction(s) is involved is not apparent at present. Given the nature of poly(vinylsulfonate), ionic binding likely plays a role, but it is not the only important factor. As shown previously (4, 18), molecular conformation as well as charge spacing influence protein-GAG binding and the inhibitory properties of GAG mimetic compounds. It may also make sense to use such compounds as inhibitors of sporozoite entry into hepatocytes (13, 37, 38, 42).
Compound 1 (and compound 2) was found to be effective both in a human in vitro malarial system and in a rodent in vivo model. This finding suggests that compound 1 inhibits a step in the invasion process that is common to multiple Plasmodium species and does not depend on the presence of a species-specific receptor-ligand combination on either the host or the parasite cell surface. The effect of compound 1 on the parasite in vitro and in vivo appears to be limited to the prevention of merozoite invasion, since merozoites that successfully invaded RBCs appeared to develop normally. It is noteworthy that compound 1 was unable to completely inhibit the invasion process in vitro, since compound 1 was able to slow the increase in the level of parasitemia but was not able to halt the infection. The incomplete inhibition may be due to the presence of a redundant, non-compound 1-related inhibitable invasion mechanism, may be due to a relatively low efficiency of blockade of merozoite entry into RBCs, allowing the eventual growth of an overwhelming merozoite population, or may reflect the fact that compound 1 has no intraerthrocytic antimalarial activity. The observation that compound 1 became less effective after 8 days in vivo suggests that a normally redundant mechanism may be present and may be being selected during the course of the infection. This possibility is being explored.
Our results also demonstrate a satisfying correlation between in vitro and in vivo activities. The compounds exerting the most significant inhibitory properties against P. falciparum in vitro proved to be the most effective against P. berghei in vivo. Our in vitro and in vivo experiments were not designed to identify the receptor-ligand combination that is being inhibited; however, observation of merozoites in culture suggests that the compounds are inhibiting the initial step of the invasion process. Further work is required to determine if the compounds interact with a merozoite surface protein, such as EBA-175, or whether they interact with an RBC receptor such as glycophorin A or B (2, 36).
It should be recognized that the samples of poly(vinylsulfate sodium salt) (compounds 1 and 2) are mixtures of oligomeric species with slightly different chain lengths and stereochemistries. Work is in progress to synthesize oligomers with defined lengths and configurations at stereogenic centers.
Our results provide significant scope for further development. Among the questions that need to be addressed are the minimum sizes of poly(vinylsulfate sodium salt)-like molecules that are effective in abrogating merozoite entry into RBCs, their possible efficacies in blocking sporozoite entry into liver cells, and whether there are specific stereoisomers among the multiple forms which poly(vinylsulfate sodium salt) can take that may be more effective than others.
Acknowledgments
We gratefully acknowledge the generous support of the Bill and Melinda Gates Foundation (to R.K.) and the able technical assistance of Michelle Ciach in the execution of this work.
REFERENCES
- 1.Achur, R. N., M. Valiyaveettil, A. Al Khalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275:40344-40356. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. H., B. K. Sim, S. A. Dolan, X. Fang, D. C. Kaslow, and L. H. Miller. 1992. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 89:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aikawa, M. 1988. Human cerebral malaria. Am. J. Trop. Med. Hyg. 39:3-10. [DOI] [PubMed] [Google Scholar]
- 4.Ancsin, J. B., and R. Kisilevsky. 1999. The heparin/heparan sulfate-binding site on apo-serum amyloid A: implications for the therapeutic intervention of amyloidosis. J. Biol. Chem. 274:7172-7181. [DOI] [PubMed] [Google Scholar]
- 5.Barragan, A., V. Fernandez, Q. J. Chen, A. Voneuler, M. Wahlgren, and D. Spillmann. 2000. The Duffy-binding-like domain 1 of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a heparan sulfate ligand that requires 12 mers for binding. Blood 95:3594-3599. [PubMed] [Google Scholar]
- 6.Chitnis, C., and M. Blackman. 2000. Host cell invasion by malarial parasites. Parasitol. Today 16:411-415. [DOI] [PubMed] [Google Scholar]
- 7.Chitnis, C. E., P. Sinnis, and L. H. Miller. 1999. The sporozoite, the merozoite, and the infected red: parasite ligands and host receptors, p. 249-285. In M. Wahlgren and P. Perlmann (ed.), Malaria: molecular and clinical aspects. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 8.Clark, D. L., S. D. Su, and E. A. Davidson. 1997. Saccharide anions as inhibitors of the malaria parasite. Glycoconjugate J. 14:473-479. [DOI] [PubMed] [Google Scholar]
- 9.Deagostini, A. I., S. C. Watkins, H. S. Slayter, H. Youssoufian, and R. D. Rosenberg. 1990. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium-antithrombin binding on cultured endothelial cells and perfused rat aorta. J. Cell Biol. 111:1293-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley, M., and L. Tilley. 1988. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 79:55-87. [DOI] [PubMed] [Google Scholar]
- 11.Foley, M., and L. Tilley. 1997. Quinoline antimalarials: mechanisms of action and resistance. Int. J. Parasitol. 27:231-240. [DOI] [PubMed] [Google Scholar]
- 12.Fraser, P. E., A. A. Darabie, and J. McLaurin. 2001. Amyloid-β interactions with chondroitin sulfate-derived monosaccharides and disaccharides—implications for drug development. J. Biol. Chem. 276:6412-6419. [DOI] [PubMed] [Google Scholar]
- 13.Fried, M., and P. E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272:1502-1504. [DOI] [PubMed] [Google Scholar]
- 14.Gowda, D. C., and C. F. Ockenhouse. 1999. Adherence of Plasmodium falciparum-infected erythrocytes to chondroitin 4-sulfate. BioScience 19:261-271. [DOI] [PubMed] [Google Scholar]
- 15.Gysin, J., B. Pouvelle, N. Fievet, A. Scherf, and C. Lepolard. 1999. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect. Immun. 67:6596-6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho, M., and N. J. White. 1999. Molecular mechanisms of cytoadherence in malaria. Am. J. Physiol. Cell. Physiol. 45:C1231-C1242. [DOI] [PubMed] [Google Scholar]
- 17.Jelinek, T., A. H. Kilian, G. Kabagambe, and F. von Sonnenburg. 1999. Plasmodium falciparum resistance to sulfadoxine/pyrimethamine in Uganda: correlation with polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes. Am. J. Trop. Med. Hyg. 61:463-466. [DOI] [PubMed] [Google Scholar]
- 18.Kerttula, Y., M. Vaara, and L. Pyhala. 1984. Effect of bacterial lipopolysaccharide on serum high density lipoprotein cholesterol in rabbits. Atherosclerosis 52:123-126. [DOI] [PubMed] [Google Scholar]
- 19.Kisilevsky, R., L. J. Lemieux, P. E. Fraser, X. Q. Kong, P. G. Hultin, and W. A. Szarek. 1995. Arresting amyloidosis in vivo using small-molecule anionic sulphonates or sulphates: implications for Alzheimer's disease. Nat. Med. 1:143-148. [DOI] [PubMed] [Google Scholar]
- 20.Makler, M. T., and D. J. Hinrichs. 1993. Measurement of lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 48:205-210. [DOI] [PubMed] [Google Scholar]
- 21.Marsh, K. 1998. Malaria disaster in Africa. Lancet 352:924.. [DOI] [PubMed] [Google Scholar]
- 22.Maubert, B., N. Fievet, G. Tami, C. Boudin, and P. Deloron. 2000. Cytoadherence of Plasmodium falciparum-infected erythrocytes in the human placenta. Parasite Immunol. 22:191-199. [DOI] [PubMed] [Google Scholar]
- 23.McCormick, C. J., C. I. Newbold, and A. R. Berendt. 2000. Sulfated glycoconjugates enhance CD36-dependent adhesion of Plasmodium falciparum-infected erythrocytes to human microvascular endothelial cells. Blood 96:327-333. [PubMed] [Google Scholar]
- 24.McCubbin, W. D., C. M. Kay, S. Narindrasorasak, and R. Kisilevsky. 1988. Circular dichroism and fluorescence studies on two murine serum amyloid A proteins. Biochem. J. 256:775-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaurin, J., T. Franklin, X. Q. Zhang, J. P. Deng, and P. E. Fraser. 1999. Interactions of Alzheimer amyloid-β peptides with glycosaminoglycans—effects on fibril nucleation and growth. Eur. J. Biochem. 266:1101-1110. [DOI] [PubMed] [Google Scholar]
- 26.McLaurin, J., D. S. Yang, C. M. Yip, and P. E. Fraser. 2000. Review: modulating factors in amyloid-β fibril formation. J. Struct. Biol. 130:259-270. [DOI] [PubMed] [Google Scholar]
- 27.Narindrasorasak, S., D. Lowery, P. Gonzalez-DeWhitt, R. A. Poorman, B. Greenberg, and R. Kisilevsky. 1991. High affinity interactions between the Alzheimer's beta-amyloid precursor proteins and the basement membrane form of heparan sulfate proteoglycan. J. Biol. Chem. 266:12878-12883. [PubMed] [Google Scholar]
- 28.Pancake, S. J., G. D. Holt, S. Mellouk, and S. L. Hoffman. 1992. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J. Cell Biol. 117:1351-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pancake, S. J., G. D. Holt, S. Mellouk, and S. L. Hoffman. 1993. Malarial sporozoites and circumsporozoite protein bind sulfated sulfated glycans: carbohydrate binding properties predicted from sequence homologies with other lectins. Parassitologia 35(Suppl):77-80. [PubMed] [Google Scholar]
- 30.Payne, D. 1987. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol. Today 3:241-246. [DOI] [PubMed] [Google Scholar]
- 31.Prudhomme, J. G., and I. W. Sherman. 1999. A high capacity in vitro assay for measuring the cytoadherence of Plasmodium falciparum-infected erythrocytes. J. Immunol. Methods 229:169-176. [DOI] [PubMed] [Google Scholar]
- 32.Rabhi-Sabile, S., M. Steiner-Mosonyi, S. Pollefeyt, D. Collen, B. Pouvelle, J. Gysin, M. C. Boffa, and E. M. Conway. 1999. Plasmodium falciparum-infected erythrocytes: a mutational analysis of cytoadherence via murine thrombomodulin. Thromb. Haemost. 81:815-821. [PubMed] [Google Scholar]
- 33.Reeder, J. C., A. F. Cowman, K. M. Davern, J. G. Beeson, J. K. Thompson, S. J. Rogerson, and G. V. Brown. 1999. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc. Natl. Acad. Sci. USA 96:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogerson, S. J., S. Novakovic, B. M. Cooke, and G. V. Brown. 1997. Plasmodium falciparum-infected erythrocytes adhere to the proteoglycan thrombomodulin in static and flow-based systems. Exp. Parasitol. 86:8-18. [DOI] [PubMed] [Google Scholar]
- 35.Rowe, A., A. R. Berendt, K. Marsh, and C. I. Newbold. 1994. Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocyte rosettes. Exp. Parasitol. 79:506-516. [DOI] [PubMed] [Google Scholar]
- 36.Sim, B. K., P. A. Orlandi, J. D. Hayes, F. W. Klotz, J. M. Carter, D. Camus, M. E. Zegans, and J. D. Chulay. 1990. Primary structure of the 175K Plasmodium falciparum erytyhrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J. Cell Biol. 111:1877-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinnis, P., and B. K. L. Sim. 1997. Cell invasion by the vertebrate stages of Plasmodium. Trends Microbiol. 5:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinnis, P., T. E. Willnow, M. R. S. Briones, J. Herz, and V. Nussenzweig. 1996. Remnant lipoproteins inhibit malaria sporozoite invasion of hepatocytes. J. Exp. Med. 184:945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone, G. C. H. 1936. Alkane-α, ο-disulfonates. J. Am. Chem. Soc. 58:488-489. [Google Scholar]
- 40.Trager, W., and J. Jensen. 1976. Human malarial parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 41.White, N. J., F. Nosten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. Kokwaro, J. Ouma, T. T. Hien, M. E. Molyneux, T. E. Taylor, C. I. Newbold, T. K. Ruebush II, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 42.Ying, P., M. Shakibaei, M. S. Patankar, P. Clavijo, R. C. Beavis, G. F. Clark, and U. Frevert. 1997. The malaria circumsporozoite protein: interaction of the conserved regions I and II-plus with heparin-like oligosaccharides in heparan sulfate. Exp. Parasitol. 85:168-182. [DOI] [PubMed] [Google Scholar]