Abstract
Inhibition of human immunodeficiency virus type 1 reverse transcriptase (RT) by both nucleoside and nonnucleoside RT inhibitors profoundly inhibits virus replication. Nucleoside RT inhibitors are known to be toxic, but there is little information regarding the toxicities of nonnucleoside RT inhibitors (NNRTI). We demonstrate that efavirenz (an NNRTI) induces caspase- and mitochondrion-dependent apoptosis of Jurkat T cells and human peripheral blood mononuclear cells. The clinical relevance of these observations is not yet clear.
The introduction of medications that target key enzymes in the virus life cycle has significantly improved the results of treating human immunodeficiency virus (HIV) infection. In particular, anti-HIV therapy which includes both reverse transcriptase (RT) inhibitors and protease inhibitors (PI) suppresses viral replication to levels below the correct limits of detection (9). Two classes of RT inhibitors are available: the nucleoside RT inhibitors (NRTIs) (including lamivudine, stavudine, zalcitabine, diadenosine, and zidovudine [AZT]) and the nonnucleoside RT inhibitors (NNRTIs) (efavirenz [EFV] [Sustiva], nevirapine [Viramune], and delavirdine [Rescriptor]). The NRTIs are incorporated into viral DNA and cause premature termination of DNA synthesis.
Unfortunately, the use of NRTIs is limited by their adverse effects: they deplete mitochondrial DNA and cytochrome c oxidase (5, 7, 14, 16), interfere with cell cycle progression, induce apoptosis (20), and are incorporated into leukocyte DNA (15). NNRTIs work differently: they bind to the catalytic site of RT and interfere with the polymerization reaction (8, 18, 19). However, few studies have examined the cellular effects of NNRTIs.
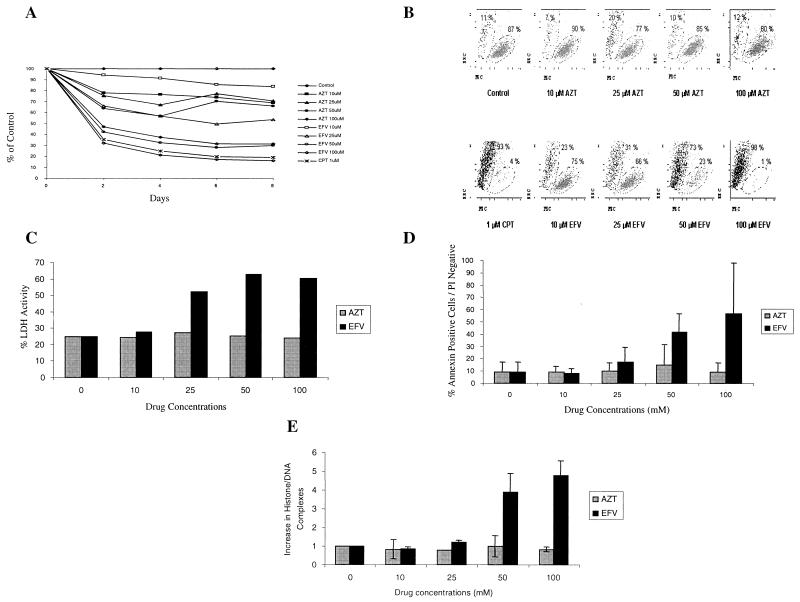
To evaluate the effect of EFV on cell survival, Jurkat T cells (ATCC) were cultured in the presence of AZT (Sigma, St. Louis, Mo.) or EFV (Dupont Pharmaceutical, Wilmington, Del.). The growth rate of Jurkat cells cultured with 50 or 100 μM of EFV was only 30% of that of the controls, whereas equimolar concentrations of AZT yielded a 50 to 70% growth rate. By forward- and side-scatter analysis using flow cytometry (Fig. 1A) (3) (Epics Altra; Coulter), 75% of the AZT-treated Jurkat cells remained in the live gate, whereas most of the EFV-treated cells shifted outside the live gate (Fig. 1B). The lactate dehydrogenase activity (LDA) (Boehringer Mannheim, Indianapolis, Ind.) of Jurkat T cells treated with 10 to 100 μM AZT (20 to 29%) was similar to the LDA of control cells (25%) (P value was not significant [NS]; n = 4). However, cells treated with 25 μM EFV had an LDA of 52% (P = 0.02; n = 4), rising to 63% for cells treated with a 50 μM concentration (P = 0.03; n = 4) (Fig. 1C).
FIG. 1.
EFV induces apoptotic cell death. (A) Jurkat T cells were seeded at 3 × 105 cells/ml and incubated with the indicated concentrations of AZT or EFV. Cell numbers were determined for every drug concentration every 2 days and expressed as a percentage of the untreated control cells. (B) Treated cells were analyzed using a Coulter EPIC Ultra flow cytometer by forward-scatter (FSC) (x axis) and side-scatter (SSC) (y axis) gating. (C) Percent LDH activity was measured after 24 h in comparison to that with camptothecin-treated cells. (D) Cell death was analyzed by flow cytometry with Annexin V-propidium iodide (PI) staining to distinguish apoptosis from necrosis. (E) Apoptosis was confirmed by histone DNA complex release.
We used flow cytometry with Annexin-V-fluorescein isothiocyanate (Pharmingen, Toronto, Canada) and propidium iodide (Sigma, St. Louis, Mo.) to distinguish apoptosis from necrosis (10). Whereas 48 h of treatment with AZT yielded low levels of apoptosis (9 to 15%) which was similar to those of untreated cells (9% [P = NS: n = 3]), EFV treatment significantly increased the apoptosis (18% at 25 μM [P < 0.01; n = 3] and 57% at 100 μM [P < 0.01; n = 3]) (Fig. 1D). The level of apoptosis (confirmed by histone-DNA complexes using the Cell Death Detection ELISAplus kit [Roche Diagnostic, Laval, Canada]) in cell extracts from AZT-treated cultures was equal to that of control cells (P = NS; n = 3), but there were four (50 μM [P = 0.02; n = 3]) to five (100 μM [P = 0.01; n = 3]) times more histone-DNA complexes in extracts from EFV-treated cells (Fig. 1E).
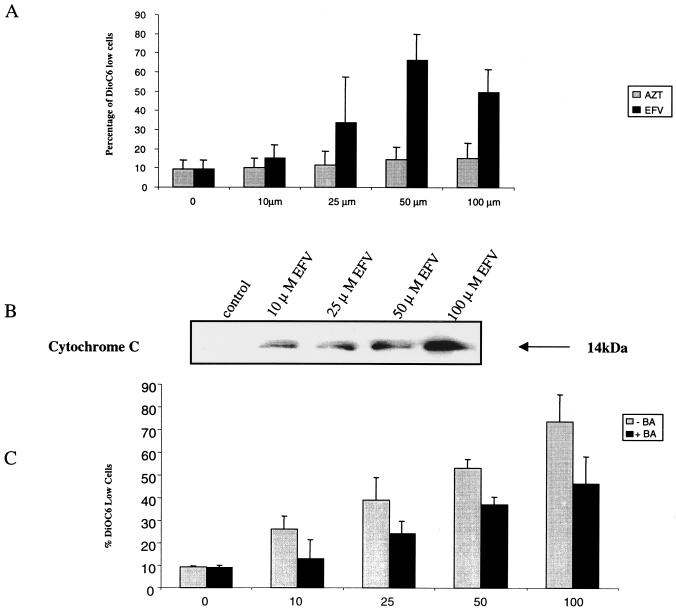
The mitochondrial changes of apoptosis include opening of the permeability transition pore complex with a subsequent loss of transmembrane potential (Δψm) and the release of cytochrome c (4). When Jurkat cells were incubated with AZT, Δψm measured (using the cationic fluorochrome DiOC6 [Molecular Probes, Eugene, Ore.]) (11) was similar to that of untreated cells (P = NS; n = 3) (Fig. 2A). By contrast, cells treated with EFV showed an increase in the percentage of DiOC6 low cells in comparison to control cells, from 34% at 25 μM (P < 0.01; n = 3) to 66% at 50 μM (P < 0.01; n = 3). Cytochrome c released from mitochondria into the cytosol was assessed by Western blot analysis (11) (anti-cytochrome c; Pharmingen, Mississauga, Canada) and revealed a dose-dependent release within 30 min of treatment with EFV at all concentrations tested (Fig. 2B). The involvement of mitochondrial Δψm in this effect was confirmed by its inhibition with 50 μM bongkrekic acid (A.G. Scientific Inc., San Diego, Calif.), which is a known permeability transition pore complex inhibitor (21) (Fig. 2C).
FIG. 2.
EFV-induced apoptosis involves mitochondrial potential loss and cytochrome c release. (A) Jurkat T cells were seeded at 3 × 105 cells/ml and treated with the indicated concentrations of AZT or EFV for 24 h. Δψm value was determined by flow cytometry by measuring the retention of the cationic fluorochrome DiOC6 in control versus treated cells. (B) Treated cells were homogenized and fractionated, and the cytosolic fraction was analyzed by Western blotting for the presence of cytochrome c. (C) Jurkat cells were treated with EFV with or without 50 μM bongkrekic acid (BA) and analyzed for Δψm value.
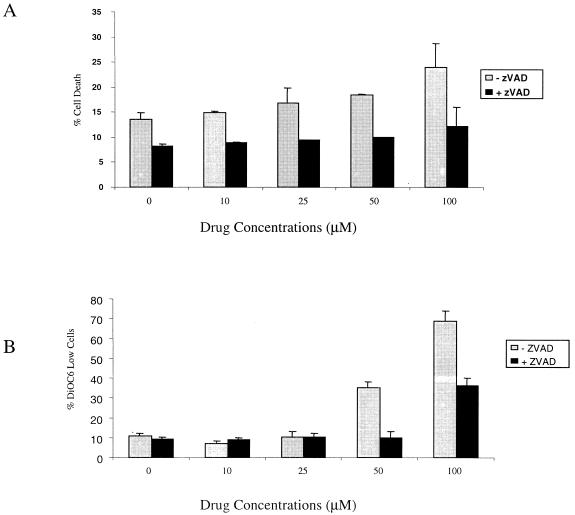
We then confirmed the caspase dependence of EFV-induced Δψm and death by culturing treated cells in the presence or absence of the pan-caspase inhibitor Z-VAD.fmk (10) (Enzyme System Products, Livermore, Calif.). Cell death induced by EFV (P = <0.01; n = 3) (Fig. 3A) and DiOC6 release (P < 0.01; n = 3) (Fig. 3B) were both significantly reduced by Z-VAD.fmk.
FIG. 3.
EFV-induced apoptosis is caspase dependent. (A) Jurkat cells were incubated with 100 μM Z-VAD.fmk for 1 h at 37°C and then treated with the indicated concentration of EFV for 4 h. Cell death was measured by Annexin-fluorescein isothiocyanate-propidium iodide staining, and the percentage of DiOC6 low cells (B) was determined.
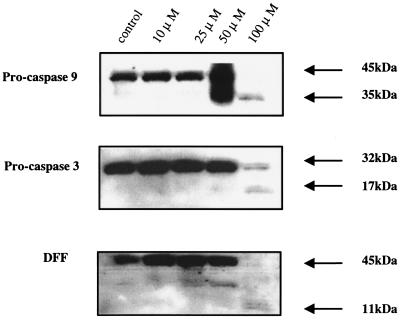
After losing transmembrane potential, cleavage of procaspase 9 and activation of caspase 3 ultimately lead to the cleavage of the DNA fragmentation factor (DFF). Using Western blot analysis (anti-caspase 9 antibody M054-3 [Medical and Biological Laboratories, Nagoya, Japan], anti-caspase 3 antibody C31720 [Transduction Laboratory, Lexington, Ky.], anti-DFF antibody sc-6866 [Santa Cruz Biotechnologies, Santa Cruz, Calif.]) to measure changes in cells treated with 100 μM EFV, we demonstrate that most of the procaspase 9 is cleaved within 30 min, releasing the active caspase 9 35-kDa fragment (Fig. 4, top) and that caspase 3 is also cleaved within 30 min following EFV treatment into a typical 17-kDa fragment which has previously been associated with caspase 3 activity (1) (Fig. 4, middle). The activity of caspase 3 is confirmed both by cleavage of DFF, a caspase 3-specific substrate (Fig. 4, bottom) and by incubation of cellular extracts with the caspase 3-specific fluorogenic substrate DEVD-AFC (17) (Enzyme System Products). In these fluorogenic release assays, AZT-treated cultures yielded little caspase 3 activity but EFV-treated cultures had high levels of caspase 3 activity (data not shown).
FIG. 4.
EFV-induced apoptosis is accompanied by caspase activation. Jurkat T cells were treated with the indicated concentrations of EFV for 30 min. Cell lysates were analyzed for the presence of procaspase 9, procaspase 3, and DFF.
To assess the potential relevance of these observations, three additional sets of experiments were performed. First, Jurkat T cells were incubated with AZT (50 μM), EFV (50 μM), or both, and apoptosis was assessed after 6 days. AZT alone resulted in 17.8% apoptosis and EFV alone resulted in 16%, whereas together they resulted in 50.1% apoptosis (P = <0.001 compared with AZT or EFV), indicating additivity or synergy of apoptotic effect. Second, Jurkat cells were incubated with either AZT (100 μM) or EFV (100 μM) for 2 h, washed, and cultured in drug-free medium. After 6 days, AZT-induced apoptosis was 19.4%, yet EFV apoptosis was 100% (P = 0.001), indicating that even short exposure to a high concentration of EFV (such as occurs at times of peak serum levels) is capable of inducing apoptosis. Third, peripheral blood mononuclear cells from four HIV-negative patients were cultured with 10, 25, 50, or 100 μM EFV or AZT and analyzed for apoptosis. Within 4 h of treatment, EFV-treated cells induced more apoptosis at all doses tested than AZT: for CD4 T cells at 10 μM, AZT = 10.25%, EFV = 14.3%, and P = NS; at 25 μM, AZT = 12.3%, EFAV = 15.25%, and P = NS, and at 50 μM, AZT = 11.5%, EFV = 45.1%, and P = 0.01). Similar results were obtained when CD8 T cells were analyzed (data not shown).
It is important to realize that the effects of EFV described here were obtained at doses as low as 10 to 25 μM, while the Cmax with 600-mg daily dosing is 12.9 ± 3.7 μM and the Cmin is 5.6 ± 3.2 μM (product insert). Further, EFV is highly protein bound, with 0.38% (range, 0.25 to 0.50%) of plasma drug remaining unbound.
Treatment of HIV with two nucleosides and either a protease inhibitor or an NNRTI effectively suppresses viral replication and improves the CD4 T cell number. Several recent studies have assessed the effect of switching from a PI-plus-dual-nucleoside regimen to one where the PI is switched to an NNRTI, either nevirapine or EFV. Although initial studies suggested that CD4 cell counts remain stable after the switch (6, 13; S. Becker, A. Rachlis, J. Gill, E. Dejesus, G. Pierone, L. Kirkland, S. Koosian, D. Farina, D. Labriola, N. Ruis, L. Bessen, and S. Villano, Abstr. 8th Conf. Retrovir. Opportunistic Infect., abstr. 20, 2001; D. Johnson and K. Squires, Abstr. 1st Int. AIDS Soc. Conf. HIV Pathog. Treatment, abstr. 423, 2001; H. Knechten, K. Sturner, C. Hohn, and P. Braun, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1532, 2000; F. Maggiolo, M. Migliorino, G. Pravettoni, M. Rizzi, S. Caprioli, and F. Suter, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1533, 2000; F. Raffi, B. Bonnet, V. Ferre, S. Leautez, V. Reliquet, C. Allavena, S. Auger, and F. Billaud, Abstr. 6th Conf. Retrovir. Opportunistic Infect., abstr. 381, 1999; R. Walli, K. Huster, J. R. Bogner, and F. D. Goebel, Abstr. 8th Conf. Retrovir. Opportunistic Infect., abstr. 672, 2001), others have noted a decline in the CD4 lymphocyte count after the switch to NNRTIs (2, 12; G. Blick, P. Greiger-Zanlungo, M. Sharfuddin, T. Garton, and E. Hatton, 1st Int. AIDS Soc. Conf. HIV Pathog. Treatment, abstr. 422, 2001; E. Martinez, J. Romeu, M. A. Garcia-Viejo, L. Cruz, J. L. Blanco, E. Negredo, B. Clotet, and J. M. Gatell, Abstr. 8th Conf. Retrovir. Opportunistic Infect., abstr. 668, 2001; M. Opravil, B. Hirschel, A. Lazzarin, J. P. Chave, H. Furrer, P. Vernazza, E. Bernasconi, M. Battegay, S. Yerly, C. Python, and L. Perrin, Abstr. 8th Conf. Retrovir. Opportunistic Infect., abstr. 476, 2000.). Further prospective studies are under way to address this question. Whereas the benefit of combination therapy, including EFV, far outweighs the known risks, further investigation may be necessary to assess these drugs' long-term effect and may influence the choice of agents for use as therapy in individuals infected with HIV.
Acknowledgments
A. A. Pilon and J. J. Lum contributed equally to this work and share first authorship.
This work is supported by grants from the Canadian Institutes on Health (CIHR), the Canadian Foundation for AIDS Research (CANFAR), and the Doris Duke Foundation to A.D.B. A.D.B. is the recipient of a Career Scientist Award from the Ontario HIV Treatment Network (OHTN) and a Premier's Research Excellence Award from the Province of Ontario. A.A.P. is the recipient of a Fellowship Award from the OHTN. J.J.L. and B.N.P. are recipients of Studentship Awards from the OHTN.
The authors are indebted to Ann Carisse for ongoing expert administrative assistance.
REFERENCES
- 1.Alam, A., L. Y. Cohen, S. Aouad, and R. P. Sekaly. 1999. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J. Exp. Med. 190:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreiro, P., V. Soriano, F. Blanco, C. Casimiro, J. J. de la Cruz, and J. Gonzalez-Lahoz. 2000. Risks and benefits of replacing protease inhibitors by nevirapine in HIV-infected subjects under long-term successful triple combination therapy. AIDS 14:807-812. [DOI] [PubMed] [Google Scholar]
- 3.Berndt, C., B. Mopps, S. Angermuller, P. Gierschik, and P. H. Krammer. 1998. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4(+) T cells. Proc. Natl. Acad. Sci. USA 95:12556-125561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner, C., and G. Kroemer. 2000. Apoptosis. Mitochondria—the death signal integrators. Science 289:1150-1151. [DOI] [PubMed] [Google Scholar]
- 5.Chariot, P., I. Monnet, and R. Gherardi. 1993. Cytochrome c oxidase reaction improves histopathological assessment of zidovudine myopathy. Ann. Neurol. 34:561-565. [DOI] [PubMed] [Google Scholar]
- 6.Clumeck, N., F. Goebel, W. Rozenbaum, J. Gerstoft, S. Staszewski, J. Montaner, M. Johnson, B. Gazzard, C. Stone, R. Athisegaran, and S. Moore. 2001. Simplification with abacavir-based triple nucleoside therapy versus continued protease inhibitor-based highly active antiretroviral therapy in HIV-1-infected patients with undetectable plasma HIV-1 RNA. AIDS 15:1517-1526. [DOI] [PubMed] [Google Scholar]
- 7.Dalakas, M. C., I. Illa, G. H. Pezeshkpour, J. P. Laukaitis, B. Cohen, and J. L. Griffin. 1990. Mitochondrial myopathy caused by long-term zidovudine therapy. N. Engl. J. Med. 322:1098-1105. [DOI] [PubMed] [Google Scholar]
- 8.Esnouf, R., J. Ren, C. Ross, Y. Jones, D. Stammers, and D. Stuart. 1995. Mechanism of inhibition of HIV-1 reverse transcriptase by non- nucleoside inhibitors. Nat. Struct. Biol. 2:303-308. [DOI] [PubMed] [Google Scholar]
- 9.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, A. Meibohm, J. H. Condra, F. T. Valentine, D. McMahon, C. Gonzalez, L. Jonas, E. A. Emini, J. A. Chodakewitz, R. Isaacs, and D. D. Richman. 2000. 3-year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann. Intern. Med. 133:35-39. [DOI] [PubMed] [Google Scholar]
- 10.Jacotot, E., K. F. Ferri, C. El Hamel, C. Brenner, S. Druillennec, J. Hoebeke, P. Rustin, D. Metivier, C. Lenoir, M. Geuskens, H. L. Vieira, M. Loeffler, A. S. Belzacq, J. P. Briand, N. Zamzami, L. Edelman, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2001. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J. Exp. Med. 193:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacotot, E., L. Ravagnan, M. Loeffler, K. F. Ferri, H. L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J. P. Briand, T. Irinopoulou, E. Daugas, S. A. Susin, D. Cointe, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez, E., I. Conget, L. Lozano, R. Casamitjana, and J. M. Gatell. 1999. Reversion of metabolic abnormalities after switching from HIV-1 protease inhibitors to nevirapine. AIDS 13:805-810. [DOI] [PubMed] [Google Scholar]
- 13.Martinez, E., M. A. Garcia-Viejo, J. L. Blanco, L. Bianchi, E. Buira, I. Conget, R. Casamitjana, J. Mallolas, and J. M. Gatell. 2000. Impact of switching from human immunodeficiency virus type 1 protease inhibitors to efavirenz in successfully treated adults with lipodystrophy. Clin. Infect. Dis. 31:1266-1273. [DOI] [PubMed] [Google Scholar]
- 14.Mhiri, C., M. Baudrimont, G. Bonne, C. Geny, F. Degoul, C. Marsac, E. Roullet, and R. Gherardi. 1991. Zidovudine myopathy: a distinctive disorder associated with mitochondrial dysfunction. Ann. Neurol. 29:606-614. [DOI] [PubMed] [Google Scholar]
- 15.Olivero, O. A., G. M. Shearer, C. A. Chougnet, A. A. Kovacs, A. L. Landay, R. Baker, A. M. Stek, M. M. Khoury, L. A. Proia, H. A. Kessler, B. E. Sha, R. E. Tarone, and M. C. Poirier. 1999. Incorporation of zidovudine into leukocyte DNA from HIV-1-positive adults and pregnant women, and cord blood from infants exposed in utero. AIDS 13:919-925. [DOI] [PubMed] [Google Scholar]
- 16.Peters, B. S., J. Winer, D. N. Landon, A. Stotter, and A. J. Pinching. 1993. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q. J. Med. 86:5-15. [PubMed] [Google Scholar]
- 17.Phenix, B. N., J. J. Lum, Z. Nie, J. Sanchez-Dardon, and A. D. Badley. 2001. Antiapoptotic mechanism of HIV protease inhibitors: preventing mitochondrial transmembrane potential loss. Blood 98:1078-1085. [DOI] [PubMed] [Google Scholar]
- 18.Spence, R. A., W. M. Kati, K. S. Anderson, and K. A. Johnson. 1995. Mechanism of inhibition of HIV-1 reverse transcriptase by nonnucleoside inhibitors. Science 267:988-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tantillo, C., J. Ding, A. Jacobo-Molina, R. G. Nanni, P. L. Boyer, S. H. Hughes, R. Pauwels, K. Andries, P. A. Janssen, and E. Arnold. 1994. Locations of anti-AIDS drug binding sites and resistance mutations in the three-dimensional structure of HIV-1 reverse transcriptase. Implications for mechanisms of drug inhibition and resistance. J. Mol. Biol. 243:369-387. [DOI] [PubMed] [Google Scholar]
- 20.Viora, M., G. Di Genova, R. Rivabene, W. Malorni, and A. Fattorossi. 1997. Interference with cell cycle progression and induction of apoptosis by dideoxynucleoside analogs. Int. J. Immunopharmacol. 19:311-321. [DOI] [PubMed] [Google Scholar]
- 21.Zamzami, N., S. A. Susin, P. Marchetti, T. Hirsch, I. Gomez-Monterrey, M. Castedo, and G. Kroemer. 1996. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 183:1533-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]