Abstract
There is a concern that there may be unregistered stocks of smallpox that can be used for bioterrorism or biological warfare. According to the WHO Advisory Committee on Variola Research, there is a need to develop strategies to treat smallpox infections should they reappear. It would also be important to have an effective drug at hand for the treatment of monkeypox disease in humans. We show here that 5-iodo-2′-deoxyuridine (IDU) is a potent inhibitor of vaccinia virus (VV) replication and that IDU inhibits VV DNA synthesis in a dose-dependent way. The in vivo protective effect of IDU was assessed in the VV tail lesion model in immunocompetent mice and in a lethal model for VV infection in SCID (severe combined immune deficiency) mice that had been infected either intranasally, intraperitoneally, or intravenously. Subcutaneous treatment with IDU at 150 and 100 mg/kg of body weight markedly reduced the number of tail lesions in immunocompetent NMRI mice. Untreated intranasally VV-infected SCID mice died at 20.8 ± 3.1 days after infection (mean ± standard deviation). Treatment with IDU (subcutaneously, 150 mg/kg/day [from day 0 to 4] and 75 mg/kg/day [from day 6 to 11]) delayed-virus induced mortality by 15 days (mean day of death ± standard deviation, 35.8 ± 6.7; P < 0.0001). This protective effect was associated with (i) an improvement of lung histology and (ii) a marked reduction in lung viral titers. IDU also delayed VV-induced mortality when mice had either been infected intraperitoneally or intravenously. Even when the start of treatment with IDU (in intraperitoneally VV-infected mice) was postponed until 2 or 4 days after infection, an important delay in virus-induced mortality was noted.
There is an increasing concern that the smallpox virus may be used for biological or terroristic purposes. All vaccination programs against smallpox were discontinued after eradication of the disease. Virtually all children and many adults are now fully susceptible to smallpox. There is only a very limited stock of vaccine available, which may not have been at all properly stored or monitored for potency. It is being studied whether diluting (1/5 or 1/10) the remaining vaccine stocks would be possible so as to increase the number of vaccines available. Novel stocks of vaccine are currently being prepared (10, 11). If smallpox were used in an act of terrorism or warfare, it could, in a highly mobile and susceptible population, cause a real catastrophe. A World Health Organization (WHO) advisory committee on variola recommended in 1999 the development of drugs to treat human smallpox infections should they reappear (press release WHO/77, 10 December 1999).
Human infection with monkeypox occurs sporadically in parts of Western and Central Africa. In 1996 and 1997 an important outbreak of monkeypox occurred in humans in the Democratic Republic of Congo (2, 13, 14, 21). Also there have recently been more outbreaks of monkeypox disease in humans. It is thus also important to have an effective drug at hand for the treatment of monkeypox disease in humans. Inhibitors of orthopoxviruses are also of interest for the treatment of molluscum contagiosum or disseminated vaccinia (17, 25; J. C. Guillaume, P. Saiag, J. Wechsler, M. C. Lescs, and J. C. Roujeau, Letter, Lancet 337:1034-1035, 1991).
We reported earlier on the potent anti-vaccinia virus (anti-VV) activity of the acyclic nucleoside phosphonate analog cidofovir, both in immunocompetent and immunodeficient mice (23). VV belongs, akin to smallpox and monkeypox viruses, to the orthopoxviruses and represents a valuable surrogate for the latter viruses (6). Our findings on the activity of cidofovir in the vaccinia model were corroborated by the recent observation that cidofovir also protects mice from a lethal aerosolized or intranasal cowpox virus or VV challenge, either when given to the mice systemically or when they are intranasally infected (3, 26, 27, 28, 29). The compound was also shown to inhibit in vitro the replication of 31 different strains of variola (according to reference 3) and to rescue nonhuman primates from large quantities (1,000 50% lethal doses) of aerosolized monkeypox (data not shown in reference 3). Cidofovir has also proven highly effective against parapoxvirus infections in vitro (22) and in vivo, in particular in a case of orf in an immunocompromised patient (12), and against molluscum contagiosum (E. G. Davies, A. Thrasher, K. Lacey, and J. Harper, Letter, Lancet 353:2042, 1999). Recently, we demonstrated that the acyclic nucleoside analogue 2-amino-7-[1,3-dihydroxy-2-propoxy)methyl]-purine (S2242) in its diacetyl ester prodrug form protected SCID mice against VV-induced mortality, when given orally (24). Here we describe the protective effect of 5′-iodo-2′-deoxyuridine (IDU) (under the trade names Herpid, Stoxil, Idoxene, and Virudox), a well-known inhibitor of herpesvirus replication, against VV infections in immunodeficient mice.
MATERIALS AND METHODS
Virus, cells, and compounds.
VV (Copenhagen strain) was obtained from the Rijksenstofinrichting (Brussels, Belgium). This virus was formerly used in Belgium as a vaccine against smallpox. Human embryonic lung (HEL) cells were obtained from the American Type Culture Collection. IDU was obtained from Sigma (St. Louis, Mo.). Cidofovir was synthesized at, and provided by, Gilead Sciences (Foster City, Calif.).
In vitro antiviral assays.
Cultures of HEL cells grown in 96-well plates were infected for 2 h at 37°C with 100 PFU of VV. Following a 2-h adsorption period, the virus inoculum was removed and the cultures were incubated at 37°C with different dilutions of the test compounds in modified Eagle medium containing 2% fetal calf serum. At 5 days postinfection, cultures were fixed with 70% ethanol and stained with a 2% Giemsa solution, and virus-induced cytopathic effects (CPEs) were recorded microscopically. The 50% effective concentration (EC50) was defined as the concentration that causes 50% inhibition of virus-induced CPE. All experiments represent mean values for two to four independent determinations.
Cytotoxic and cytostatic determinations.
Cytotoxicity was assessed in confluent HEL cell cultures that had been incubated for 5 consecutive days with serial dilutions of the test compounds and was monitored microscopically or by means of the MTS method (Promega). The tetrazolium salt MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium], when applied to living cells, is converted to a colored formazan. The cytostatic potential of the compounds was assessed in exponentially growing HEL cells. Briefly, HEL cells were seeded in 96-well plates at a density of 4,000 cells per well. At 6 to 8 h after seeding, the cells were incubated with serial dilutions of the test compounds. Cultures were then incubated for 3 days, at which time cell proliferation was determined by the MTS method (Promega). All experiments are mean values for two to four independent determinations.
Determination of viral DNA levels.
Confluent cultures of HEL cells grown in 25-cm2 culture flasks were inoculated with VV at a multiplicity of infection of 0.2 for 2 h. After the virus had been removed, cultures were either treated with the test compounds or left untreated. At day 5 postinfection, at which time untreated infected cultures showed 100% CPE, cells were collected, total cellular DNA was extracted (Qiagen blood kit), and 10 μg of heat denatured total cellular DNA was blotted onto a nylon membrane (Hybond-N; Amersham). Following UV cross-linking, prehybridization was carried out for 1 h at 42°C. A digoxigenin-11-dUTP-labeled VV-specific probe was generated as described previously (24). Hybridization was carried out for 18 h at 42°C with 30 ng of the digoxigenin-11-dUTP labeled probe per ml. Following washes at high and low stringency, filters were incubated with antidigoxigenin antibody conjugated with alkaline phosphatase (Boehringer Mannheim). Detection of chemiluminescence was performed by standard methods, and films were scanned densitometrically.
Inoculation, treatment, and evaluation.
SCID mice (bred at the Rega Institute under specific-pathogen-free conditions) weighing about 15 g, received inoculations intranasally (following sedation), intravenously (in the tail vein), or intraperitoneally with, respectively, 20, 50, or 200 μl of VV (stock of ∼105 PFU/ml). IDU or (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) (cidofovir) (for treatment schedules, see Figures and Tables) was administered by subcutaneous injection (in 0.2 ml of phosphate-buffered saline). Mortality was recorded daily. Naval Medical Research Institute (NMRI) mice (Harlan) weighing 17 to 20 g were inoculated in the tail vein with 4 × 103 PFU of VV. Animals were treated by subcutaneous injection with IDU or cidofovir. The number of tail lesions (vesiculae or spots) was recorded at days 6 and 8 postinfection. Statistically significant differences in the mean day of death (MDD) (for SCID mice) or the number of tail lesions (for NMRI mice) was assessed by means of Student's t test. The animal experiments were approved by the Ethical Committee on Vertebrate Animals of the University of Leuven.
Determination of viral titers in lungs.
At day 5, 10, 15, 20, or 28 postinfection, lungs from (for each time point) three infected animals that had either been treated with IDU or HPMPC or that had been left untreated were dissected aseptically. Tissue homogenates (10%, wt/vol) were prepared in 2% modified Eagle medium and were titrated (fivefold serial dilutions) on HEL cells. Virus-induced CPE was recorded 5 days later.
Histology.
SCID mice (∼15 g) that had been inoculated intranasally with VV and that had either been left untreated or that were treated subcutaneously with IDU (150 mg/kg/day from day 0 to 4 and 75 mg/kg/day from day 7 to 11) or with cidofovir (at 25 mg/kg/day from day 0 to 4 and from day 7 to 11) were killed by ether anesthesia; lungs were dissected, fixed (in Bouin), embedded in paraffin, and sectioned. Sections were stained with hematoxylin-eosin.
RESULTS
In vitro anti-VV activity of IDU.
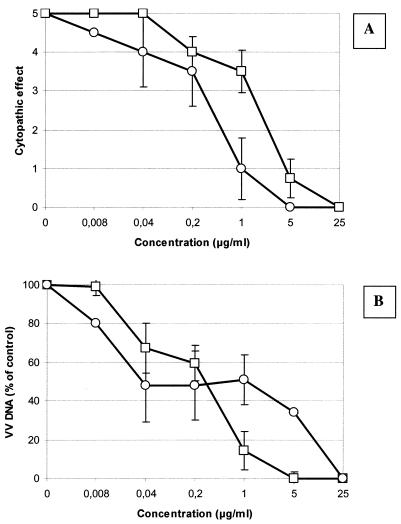
The in vitro anti-VV activities of IDU and HPMPC in HEL cells were compared. As can be derived from Fig. 1A, IDU efficiently inhibited VV-induced CPE formation in fibroblasts with an EC50 of 0.4 μg/ml; the EC50 of HPMPC was 2.6 μg/ml. Viral DNA synthesis was monitored in parallel (Fig. 1B). A dose-dependent inhibition of VV DNA synthesis was observed, with EC50 values of 0.3 μg/ml for both IDU and HPMPC. The concentration that causes a ≥20% reduction in cell metabolism (i.e., mitochondrial activity) as assessed by the MTS method was >100 μg/ml for both IDU and HPMPC. Cytostatic effects of HPMPC and IDU were assessed in uninfected exponentially growing HEL cells. The 50% cytostatic concentrations of IDU and HPMPC were 69 ± 36 and 42 ± 15 μg/ml, respectively (means ± standard deviations). IDU and HPMPC thus yielded comparable selectivity indices (for IDU, 230; for HPMPC, 140) when inhibition of viral DNA synthesis was used as a parameter for drug efficacy.
FIG. 1.
In vitro anti-VV activity of IDU or HPMPC, as assessed by recording virus-induced CPE (upper panel) or viral DNA synthesis (lower panel). Symbols: ○, IDU; □, HPMPC. All data are mean values ± standard deviations (error bars) for two to four independent determinations.
Effect of IDU on VV-induced tail lesions in NMRI mice.
Immunocompetent NMRI mice received treatment with IDU at 150, 100, or 75 mg/kg/day from day 0 to 5 postinfection. Typical tail lesions (first spots, then vesiculae) developed in control animals. The protective effects of IDU and HPMPC were assessed at day 6 and 8 postinfection. On day 6 postinfection, no vesiculae had developed on the tail of mice that had been treated with IDU at 150 or 100 mg/kg/day, whereas all untreated animals showed large numbers of lesions on the tail at that time. IDU still resulted in a marked reduction in the number of vesiculae on day 8 postinfection (Table 1). Treatment with IDU at 150 or 100 mg/kg/day resulted in a reduction of body weight of, respectively, 17 and 6% on day 8 postinfection (compared to the body weight at the start of the experiment). Yet, the general condition of the animals appeared to be all right.
TABLE 1.
Effect of IDU on VV-induced tail lesion formation in intravenously infected NMRI mice
| IDU treatmenta (mg/kg/day) | Effect of treatment on:
|
|||||
|---|---|---|---|---|---|---|
| Day 6 postinfection
|
Day 8 postinfection
|
|||||
| Vesiculaeh | Spotsh | % BDWc | Vesiculae | Spots | % BDW | |
| None | 20 ± 7.6 (8/8)b | —d | 103 | 18.3 ± 7.3 (8/8) | — | 104 |
| 150 | — | 3.6 ± 1.5 (5/5) | 85 | 6.4 ± 3.3f (5/5) | — | 83 |
| 100 | — | 2.6 ± 1.6 (4/5) | 94 | 11 ± 3.3g (4/5) | — | 94 |
| 75 | 20 ± 6.4e (4/5) | 4 (1/5) | 102 | 17.2 ± 8.3e (5/5) | — | 96 |
Treatment was started 2 h postinfection and was continued for the next 4 days. Compounds were administered subcutaneously twice a day.
Values in parentheses indicate the number of animals with either vesiculae or spots on their tails/number of animals treated.
% BDW, percent body weight, compared to the body weight at the start of the experiment.
—, None detectable.
Not significant (P > 0.05) compared to the untreated control.
P < 0.005 compared to the untreated control.
P < 0.05 compared to the untreated control.
Number of vesiculae or spots are mean values ± standard deviations.
Effect of IDU on VV-induced morbidity and mortality in intranasally infected SCID mice.
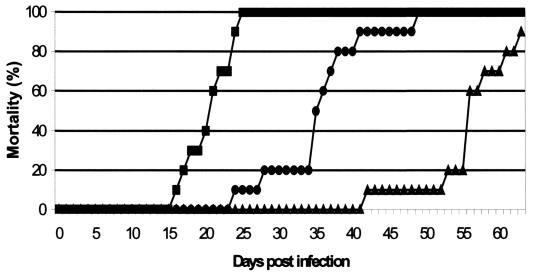
SCID mice that had been infected intranasally became sick (ruffled fur, cachexia) at about day 14 postinfection and died at 20 days postinfection (MDD ± standard deviation, 20.8 ± 3.1) (Fig. 2). When IDU was administered subcutaneously (150 mg/kg/day from day 0 to 4 and 75 mg/kg/day from day 7 to 11), virus-induced mortality was delayed by 15 days (MDD, 35.8 ± 6.7; p < 0.00005). This particular dosing schedule allowed an “aggressive” induction dose with IDU, followed by a lower dosage that was also effective but that had less of an effect on body weight. HPMPC, which was included as a reference compound at 25 mg/kg/day (same treatment schedule as for IDU), delayed virus-induced mortality by 38 days (MDD, 55 ± 5.9).
FIG. 2.
Effect of IDU or HPMPC on VV-induced mortality in intranasally infected SCID mice. Data are mean values from two independent experiments. IDU was administered subcutaneously at 150 mg/kg/day from day 0 to 4 and at 75 mg/kg/day from day 7 to 11, and HPMPC was administered at 25 mg/kg/day from day 0 to 4 and from day 7 to 11. Symbols: ▪, untreated controls (n = 10); •, IDU-treated mice (n = 10); ▴, HPMPC-treated mice (n = 9).
In a parallel set of experiments viral titers were measured in the lungs of treated and untreated animals at 5, 10, 15, 20, and 28 days postinfection (lungs of three animals were pooled) (data not shown). Titers increased steadily in the lungs of untreated animals from day 5 to 15 postinfection (2.0 × 104, 6.0 × 104, and 2.4 × 105 PFU/g tissue, respectively). None of the untreated animals survived after day 20. In the IDU-treated animals, viral titers were below the detection limit for the first 15 days. Some infectious virus was detected (∼250 PFU/g tissue) at day 20. At day 28 postinfection viral titers in the lungs were comparable (2.8 × 105 PFU/ml) to titers in the lungs of untreated animals at day 15 postinfection. No virus was detectable in HPMPC-treated animals up to 28 days postinfection.
Histologically, the lungs of intranasally infected animals (at 18 days postinfection) showed a patchy pattern of mild infiltration of alveolar walls by neutrophils. Exudation of leukocytes into alveolar spaces was minimal or even absent. Some distal bronchioli exhibited an infiltration of the mucosa with neutrophils. Lungs of IDU- or HPMPC-treated animals (at 18 day postinfection) were histologically indistinguishable from lungs of uninfected animals.
Effect of IDU on VV-induced mortality in intraperitoneally or intravenously infected SCID mice.
SCID mice that had been inoculated intravenously died at about 18 days postinfection (Table 2). Mortality of mice that had been treated with IDU at 100 mg/kg/day from day 0 to 4 postinfection and at 50 mg/kg/day from day 7 to 11 postinfection was delayed by 9 days. A similar treatment schedule with IDU at 50 mg/kg/day resulted in some delay in virus-induced mortality, although this was statistically not significant (P = 0.07).
TABLE 2.
Effects of IDU and HPMPC on VV-induced mortality in SCID mice that had been infected intravenously or intraperitoneally
| Condition and treatmenta | No. of survivorse | MDD ± SD (P) |
|---|---|---|
| Intravenous infection | ||
| None | 0 | 18.0 ± 4.1 |
| IDU (day 0-4, 100 mg/kg/day; day 7-11, 50 mg/kg/day) | 0 | 27.4 ± 7.4 (<0.05) |
| IDU (day 0-4, 50 mg/kg/day; day 7-11, 50 mg/kg/day) | 0 | 25.6 ± 6.8 (NSc) |
| HPMPC (day 0-4, 25 mg/kg/day day 7-11, 25 mg/kg/day) | 0 | 52.2 ± 5.4 (<0.0001) |
| Intraperitoneal infection | ||
| None | 0 | 33.6 ± 11.6 |
| IDU (day 1-2, 250 mg/kg/day; weekd 2, 3, 4, 5, 100 mg/kg/day) | 4b | 3 |
| IDU (week 1, 100 mg/kg/day; weekd 2, 3, 4, 5, 50 mg/kg/day) | 0 | 60.4 ± 20.5 (<0.05) |
Animals were treated subcutaneously with the indicated treatment regimen.
Survivors were sacrificed at 135 days postinfection, at which time their organs were found to be free of virus.
NS, P = 0.07.
“Week” means 5 consecutive days of treatment followed by cessation of therapy for 2 days.
Fire SCID mice per treatment group.
SCID mice that had been infected intraperitoneally, and that were left untreated, died about 1 month postinfection (MDD, 33.6 ± 11.6) (Table 2). Treatment with IDU at 100 mg/kg/day, from day 0 to 4 and at 50 mg/kg/day from day 7 to 11, day 14 to 18, day 21 to 25, and day 28 to 32 delayed virus-induced mortality by almost 1 month (MDD 60.4 ± 20.6). Four out of five animals that had been treated with IDU at 250 mg/kg from day 0 to 2 and at 100 mg/kg from day 7 to 11, day 14 to 18, day 21 to 25, and day 28 to 32 survived the infection until 135 days postinfection, at which time they were sacrificed. No virus was recovered from their organs at that time.
Effect of delayed start of treatment with IDU on VV-induced mortality in intraperitoneally infected SCID mice.
Even when start of treatment with IDU in intraperitoneally infected animals was delayed for 2 or 4 days postinfection, a significant delay in virus-induced mortality was still noted, although the protective effect was less pronounced than when treatment was initiated at the day of infection (Table 3). When the start of treatment was delayed for 7 days postinfection, no significant delay in virus-induced mortality was noted.
TABLE 3.
Effect of delayed start of treatment with IDU on VV-induced mortality in SCID mice
| Treatmenta | No. of survivorsb | MDD ± SD (P) |
|---|---|---|
| Placebo | 0 | 30.4 ± 7.3 |
| IDU (day 0-4 and day 7-11) | 2c | 163 ± 5.1 (<0.0001) |
| IDU (day 2-6 and day 9-13) | 0 | 43.8 ± 6.8 (<0.02) |
| IDU (day 4-8 and day 11-15) | 0 | 40.4 ± 6.2 (<0.05) |
| IDU (day 7-11 and day 14-18) | 0.5 | 37.2 ± 6.3 (NSd) |
SCID mice were infected intraperitoneally and were treated subcutaneously with IDU at 75 mg/kg/day for 2 periods of 5 consecutive days (as indicated). Treatment was initiated at either 2 h postinfection (day 0), or 2, 4, or 7 days postinfection.
Five SCID mice per treatment group.
Survivors were sacrificed at 179 days postinfection, at which time their organs were found to be free of virus.
NS, P > 0.05
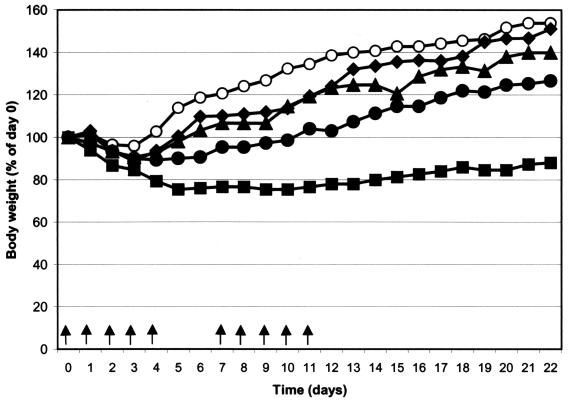
Effect of IDU treatment on growth of mice.
We documented the effect of treatment with IDU on the growth of young SCID mice (Fig. 3). Mice received treatment schedules as used in the experiment depicted in Fig. 2 and in the experiments presented in Tables 2 and 3. Treatment with IDU at 150 mg/kg/day (day 0 to 4) and 75 mg/kg/day (day 7 to 11) had an important effect on the growth of the animals. All mice survived, however, and their body weight slowly increased following cessation of therapy. Lower doses of drugs that still proved antivirally active (Fig. 2; Tables 1 and 2) were well tolerated and resulted in a less-pronounced growth retardation.
FIG. 3.
Change in body weight of SCID mice (five mice per condition) that had been treated twice daily by subcutaneous injection with IDU using the following treatment schedules: IDU at 150 mg/kg/day on days 0 to 4 and 75 mg/kg/day on days 7 to 11 (▪), IDU at 100 mg/kg/day on days 0 to 4 and 50 mg/kg/day on days 7 to 11 (•), IDU at 75 mg/kg/day on days 0 to 4 and on days 7 to 11 (▴); and IDU at 50 mg/kg/day on days 0 to 4 and on days 7 to 11 (⧫). Data for animals that were left untreated are also indicated (○).
DISCUSSION
Until the introduction of vaccines in the early 1800s, poxviruses historically caused serious disease in humans. The last documented case of smallpox occurred in 1977 in Somalia, and the WHO declared that smallpox had been eradicated as of 1980. Before that time the virus had killed millions of people. The public health threat of poxviruses has recently surfaced with (i) the fear for terrorist attacks with smallpox and (ii) the observation of monkeypox infections in humans, especially in the period from 1996 to 1997 in the Democratic Republic of Congo (formerly Zaire) (14). Monkeypox infection in humans is associated with signs and symptoms similar to those of smallpox, including the development of a pustular rash that is clinically indistinguishable from smallpox. The infection, however, is far less lethal than smallpox (4, 13, 21).
Starting in 1977, the number of laboratories holding stocks of the variola virus was reduced until all known stocks were consolidated in only two WHO collaborating centers: the Centers for Disease Control and Prevention in Atlanta, Ga., and the State Research Center of Virology and Biotechnology (VECTOR) laboratories in Kotsovo, near Novosibirsk, Russia. Although outlawed by the Biological Weapons Convention in 1972, there is a serious concern that there may be unregistered stocks of smallpox that can be used for bioterrorism or biological warfare. A smallpox outbreak occurring today in a highly mobile and susceptible population would likely spread widely before effective measures could be taken (4; C. Hooper, Letter, N. Engl. J. Med. 339:2027-2028, 1998). Since there is no approved treatment for smallpox the WHO expert committee on orthopoxvirus infection has recommended that specific antivirals for the treatment of poxviruses should be developed before the destruction of the (officially remaining) stocks of variola (press release WHO/77, 10 December 1999).
We previously reported the activity of some nucleoside phosphonate analogs against VV replication. The prototype compound of this family, (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine (HPMPA), was found to exhibit a broad-spectrum activity against a wide variety of DNA viruses, including VV (8), and inhibited the development of tail lesions caused by VV (7). Cidofovir (HPMPC), which is the cytosine counterpart of HPMPA and which has been approved for the treatment of cytomegalovirus retinitis in immunocompromised patients (i.e., patients with AIDS), has an activity spectrum comparable to that of HPMPA but has a more-favorable toxicity profile in vitro and in vivo (i.e., in mice). We have demonstrated that cidofovir possesses potent protective activity against a lethal VV infection in SCID mice (23). Our observations were corroborated by the findings of Bray and colleagues who demonstrated that cidofovir is also effective in the treatment of lethal aerosolized or intranasal cowpox virus infections in mice (3). In the same report these authors revealed (their unpublished data) that cidofovir rescued monkeys that had received large quantities (1,000 50% lethal doses) of aerosolized monkeypox and that the compound was active in vitro against several different strains of smallpox. The antipoxvirus activity of cidofovir was also further documented in murine models (27, 28, 29). Recently, cidofovir-resistant strains of camelpox, cowpox, and monkeypox viruses and VV were generated (30). Cidofovir has been shown to result in complete regression of lesions associated with poxviruses in humans, i.e., molluscum contagiosum lesions (19, 31; V. Ibarra, J. R. Blanco, J. A. Oteo, and L. Rosel, Letter, Acta Derm. Venereol. 80:315-316, 2000) and orf (ecthyma infections) in an immunodeficient patient (12).
Recently, we demonstrated that the acyclic nucleoside analogue 2-amino-7-[1,3-dihydroxy-2-propoxy)methyl]purine (S2242) and its diacetylated oral prodrug form HOE961 (the latter when given orally) elicit potent activity against VV infections in both immunocompetent and SCID mice (24). There were no obvious side effects associated with this treatment. A compound that can be given orally offers, particularly for the treatment of poxvirus infections, an advantage over compounds that must be given intravenously. In this context it is of interest to mention that alkoxyalkyl esters of cidofovir and cyclic cidofovir that may have an improved oral bioavailability profile showed enhanced in vitro inhibition of orthopoxvirus replication (16).
We demonstrate here that the antiherpetic compound IDU (under the trade names Herpid, Stoxil, Idoxene, and Virudox) is able to cause an important delay in VV-induced mortality in SCID mice infected either intranasally, intraperitoneally, or intravenously. The protective activity of IDU (and that of HPMPC) was also reflected by the fact that lung histology in drug-treated infected animals was normal (at 18 days postinfection), whereas focal anomalies (similar to those described in reference 20) were noted in the untreated mice. The lung pathology as seen here in the untreated mice is not as severe as the pathology reported in the vaccinia model employed by Smee and colleagues (27, 28). This may be explained by the fact that in the present study (i) SCID mice were used, which lack functional T and B cells and which may, therefore, have less immunopathological involvement (BALB/c mice were used by Smee and colleagues) and (ii) a strain of virus was used that was different from that used by Smee and colleagues. Also in the cowpox model, lung pathology was reported to be more severe than that observed here (18).
The protective activity of IDU in a nonlethal VV pox tail lesion model was already reported 25 years ago (9). It has, however, remained unknown whether IDU would cause protection against lethal orthopoxvirus infections. Other compounds, such as trifluorothymidine and arabinofuranosyl cytosine have also been reported to cause protection in the VV tail lesion model (9) or in the VV keratitis model in rabbits (15). We were, however, not able to demonstrate any protective effect with either arabinofuranosyl cytosine or trifluorothymidine against lethal VV infections in SCID mice (data not shown).
The use of IDU for the treatment of herpesvirus infections is restricted to topical use because the compound was found to be too toxic for intravenous administration (1). However, unlike for herpesvirus infections, there is only one compound, i.e., cidofovir, that could possibly be used for the treatment of infections with variola. In addition, as mentioned above, it has now been shown that cidofovir-resistant strains can be generated (30). Deliberate release of cidofovir-resistant variola strains would leave us without option for treatment Although one should aim to use compounds that cause as little as possible adverse effects for the treatment of infections with variola, the fact that the fatality rate associated with smallpox may be estimated at 10 to 50% (or even higher in immunocompromised patients) may possibly justify the administration of a relatively toxic compound for a short period of time during the acute phase of the infection. Indeed, the fatality rate of smallpox is much higher than that of many oncological diseases for which patients receive treatment with (relatively) toxic substances. Further studies are warranted to determine whether IDU causes protection against other orthopoxvirus infections (i.e., smallpox, cowpox, monkeypox, camelpox) in vitro and in vivo.
Acknowledgments
This work was supported by the Geconcerteerde Onderzoeksacties (GOA 00/12). J. Neyts is supported by the Fonds voor Wetenschappelijk Onderzoek (FWO)-Vlaanderen.
We thank W. Zeegers and M.-H. Stuyck for expert technical assistance and D. Brabants, I. Aerts, and C. Callebaut for dedicated editorial help.
REFERENCES
- 1.Alford, C. A. Jr., and Whitley, R. J. 1976. Treatment of infections due to Herpesvirus in humans: a critical review of the state of the art. J. Infect. Dis. 133(Suppl.):A101-A108. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1997. Human monkeypox—Kasai Oriental, Democratic Republic of Congo, February 1996-October 1997. Morb. Mortal. Wkly. Rep. 46:1168-1171. [PubMed] [Google Scholar]
- 3.Bray, M., M. Martinez, D. F. Smee, D. Kefauver, E. Thompson, and J. W. Huggins. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 181:10-19. [DOI] [PubMed] [Google Scholar]
- 4.Breman, J. G., and D. A. Henderson. 1998. Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. N. Engl. J. Med. 339:556-559. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, J. 2001. Vaccines for biodefense: a system in distress. Science 294:498-501. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq, E. 2001. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 14:382-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Clercq, E., A. Holý, and I. Rosenberg. 1989. Efficacy of phosphonyl-methoxyalkyl derivatives of adenine in experimental herpes simplex virus and vaccinia virus infections in vivo. Antimicrob. Agents Chemother 33:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Clercq, E., A. Holý, I. Rosenberg, T. Sakuma, J. Balzarini, and P. C. Maudgal. 1986. A novel selective broad-spectrum anti-DNA virus agent. Nature 323:464-467. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq, E., M. Luczak, D. Shugar, P. F. Torrence, J. A. Waters, and B. Witkop. 1976. Effect of cytosine, arabinoside, iododeoxyuridine, ethyldeoxyuridine, thiocyanatodeoxy-uridine, and ribavirin on tail lesion formation in mice infected with vaccinia virus. Proc. Soc. Exp. Biol. Med. 151:487-490. [DOI] [PubMed] [Google Scholar]
- 10.Enserink, M., and R. Stone. 2002. Public health. Dead virus walking. Science 295:2001-2005. [DOI] [PubMed] [Google Scholar]
- 11.Enserink, M. 2002. Smallpox vaccines. New cache eases shortage worries. Science 296:25-27. [DOI] [PubMed] [Google Scholar]
- 12.Geerinck, K., G. Lukito, R. Snoeck, R. De Vos, E. De Clercq, Y. Vanrenterghem, H. Degreef, and B. Maes. 2001. A case of human orf in immunocompromised patient treated successfully with cidofovir cream. J. Med. Virol. 64:543-549. [DOI] [PubMed] [Google Scholar]
- 13.Heymann, D. L., M. Szczeniowski, and K. Esteves. 1998. Re-emergence of monkeypox in Africa: a review of the past six years. Br. Med. Bull. 54:693-702. [DOI] [PubMed] [Google Scholar]
- 14.Hutin, Y. J., R. J. Williams, P. Malfait, R. Pebody, V. N. Loparev, S. L. Ropp, M. Rodriguez, J. C. Knight, F. K. Tshioko, A. S. Khan, M. V. Szczeniowski, and J. J. Esposito. 2001. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 7:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyndiuk, R. A., S. Seideman, and J. M. Leibsohn. 1976. Treatment of vaccinial keratitis with trifluorothymidine. Arch. Ophthalmol. 94:1785-1786. [DOI] [PubMed] [Google Scholar]
- 16.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kesson, A. M., J. K. Ferguson, W. D. Rawlinson, and A. L. Cunningham. 1997. Progressive vaccinia treated with ribavirin and vaccinia immune globulin. Clin. Infect. Dis. 25:911-914. [DOI] [PubMed] [Google Scholar]
- 18.Martinez, M. J., M. P. Bray, and J. W. Huggins. 2000. A mouse model of aereosol-transmitted orthopoxviral disease: morphology of experimental aerosol-transmitted orthopoxviral disease in cowpox virus-BALB/c mouse system. Arch. Pathol. Lab. Med. 124:362-377. [DOI] [PubMed] [Google Scholar]
- 19.Meadows, K. P., S. K. Tyring, A. T. Pavia, and T. M. Rallis. 1997. Resolution of recalcitrant molluscum contagiosum virus lesions in human immunodeficiency virus-infected patients treated with cidofovir. Arch. Dermatol. 133:987-990. [PubMed] [Google Scholar]
- 20.Montasir, M., E. R. Rabin, and C. A. Phillips. 1966. Vaccinia pneumonia in mice. A light and electron microscopic and viral assay study. Am. J. Pathol. 48:877-895. [PMC free article] [PubMed] [Google Scholar]
- 21.Mukinda, V. B., G. Mwema, M. Kilundu, D. L. Heymann, A. S. Khan, J. J. Esposito, et al.. 1997. Re-emergence of human monkeypox in Zaire in 1996. Lancet 349:1449-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nettleton, P. F., J. A. Gilray, H. W. Reid, and A. A. Mercer. 2000. Parapoxviruses are strongly inhibited in vitro by cidofovir. Antivir. Res. 48:205-208. [DOI] [PubMed] [Google Scholar]
- 23.Neyts, J., and E. De Clercq. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)-cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 41:242-246. [DOI] [PubMed] [Google Scholar]
- 24.Neyts, J., and E. De Clercq. 2001. Efficacy of 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)-purine for the treatment of vaccinia virus (orthopoxvirus) infections in mice. Antimicrob. Agents Chemother. 45:84-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redfield, R. R., D. C. Wright, W. D. James, T. S. Jones, C. Brown, and D. S. Burke. 1987. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 316:673-676. [DOI] [PubMed] [Google Scholar]
- 26.Smee, D. F., K. W. Bailey, and R. W. Sidwell. 2000. Treatment of cowpox virus respiratory infections in mice with ribavirin as a single agent or followed sequentially by cidofovir. Antivir. Chem. Chemother. 11:303-309. [DOI] [PubMed] [Google Scholar]
- 27.Smee, D. F., K. W. Bailey, and R. W. Sidwell. 2001. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antivir. Chem. Chemother. 12:71-76. [DOI] [PubMed] [Google Scholar]
- 28.Smee, D. F., K. W. Bailey, M.-H. Wong, and R. W. Sidwell. 2000. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antivir. Res. 47:171-177. [DOI] [PubMed] [Google Scholar]
- 29.Smee, D. F., K. W. Bailey, M.-H. Wong, and R. W. Sidwell. 2001. Effects of cidofovir on the pathogenesis of a lethal vaccinia virus respiratory infection in mice. Antivir. Res. 52:55-62. [DOI] [PubMed] [Google Scholar]
- 30.Smee, D. F., R. W. Sidwell, D. Kefauver, M. Bray, and J. W. Huggins. 2002. Characterization of wild-type and cidofovir-resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses. Antimicrob. Agents Chemother. 46:1329-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toro, J. R., L. V. Wood, N. K. Patel, and M. L. Turner. 2000. Topical cidofovir: a novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus 1. Arch. Dermatol. 136:983-985. [DOI] [PubMed] [Google Scholar]