Abstract
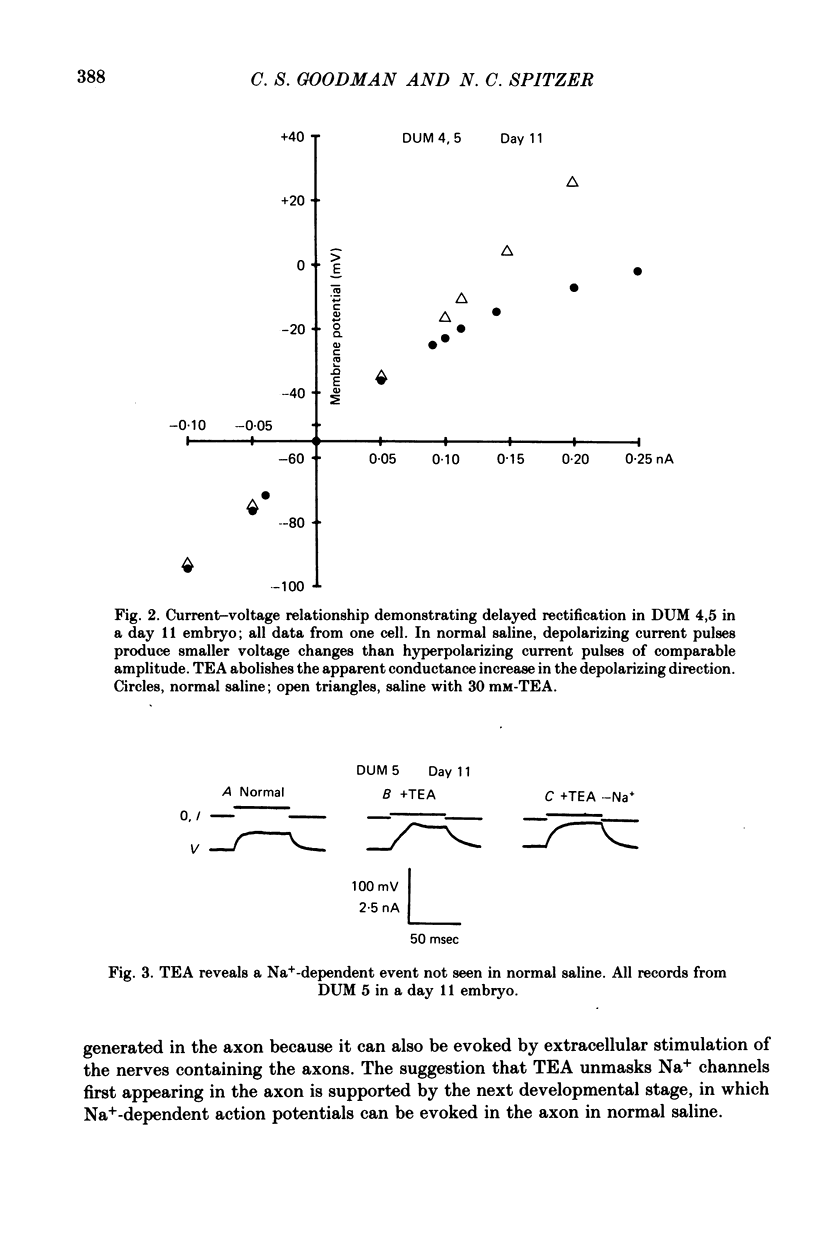
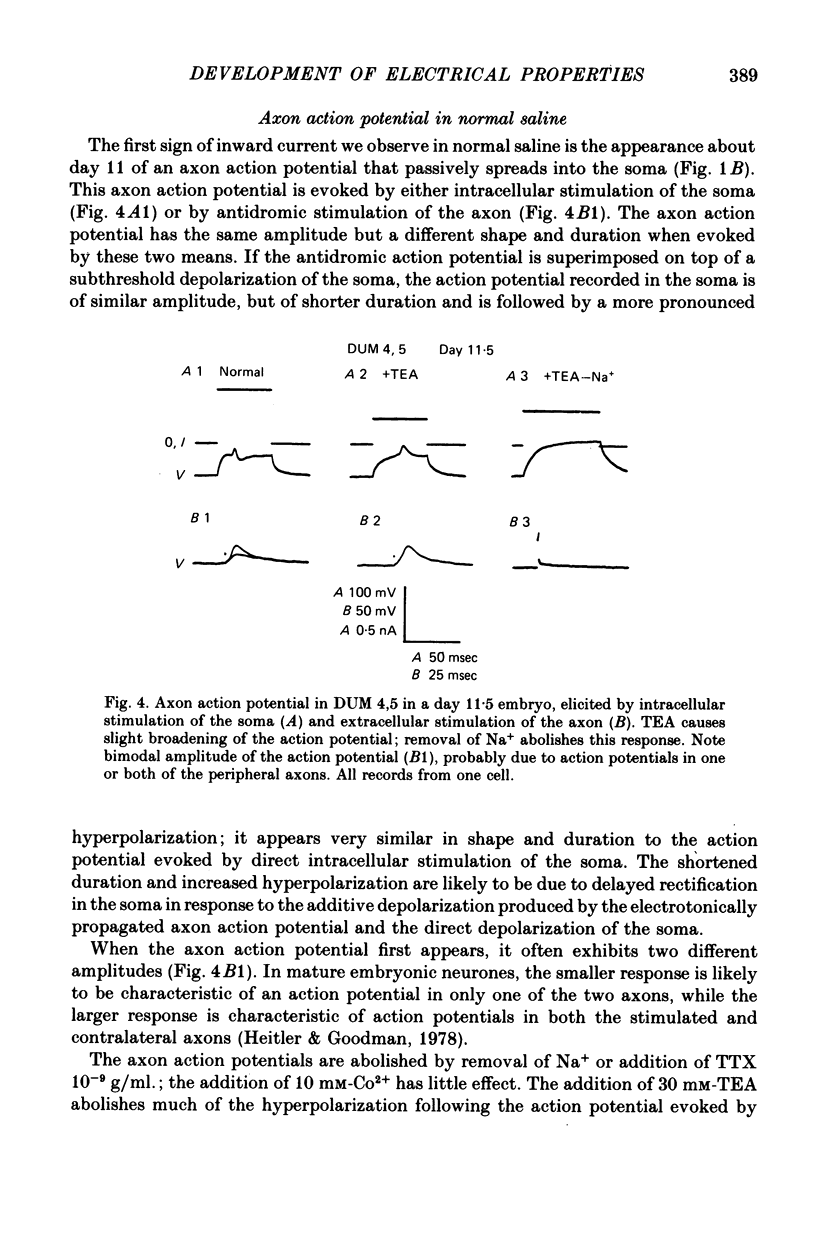
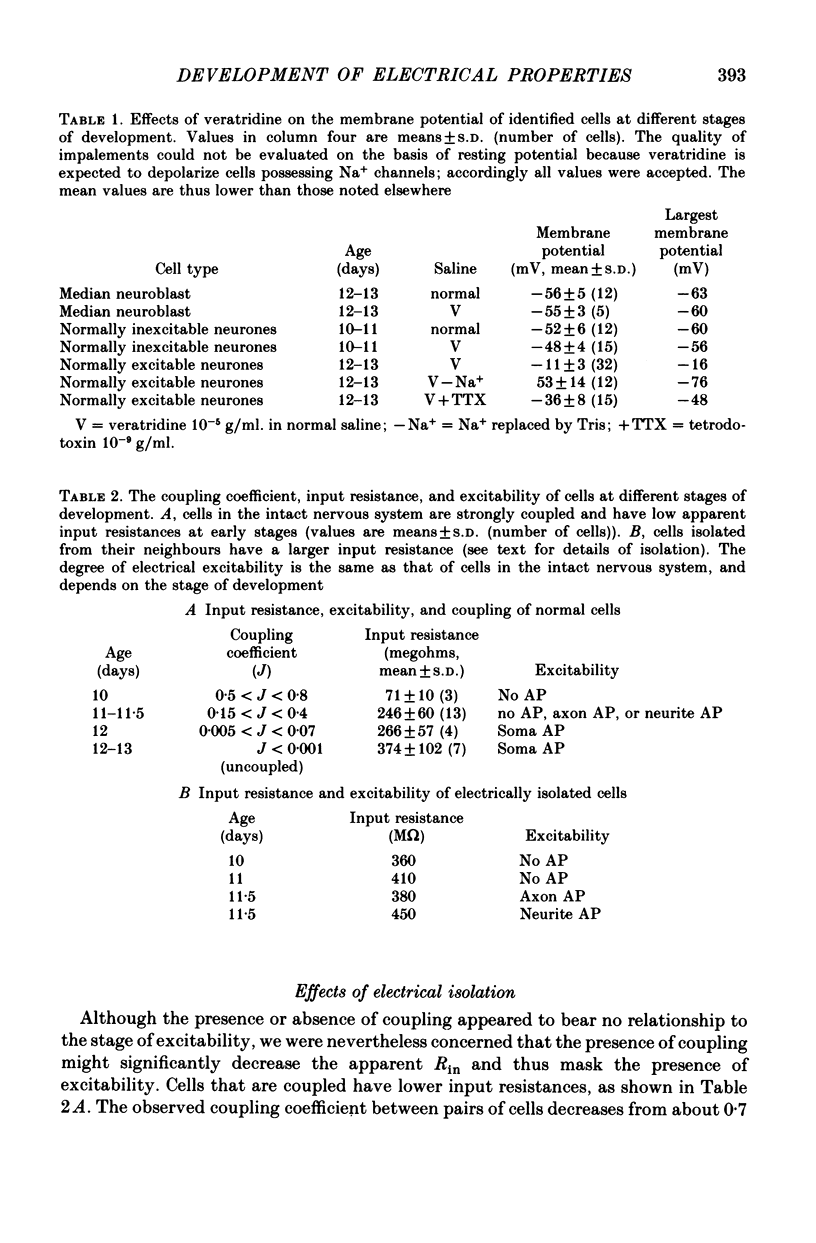
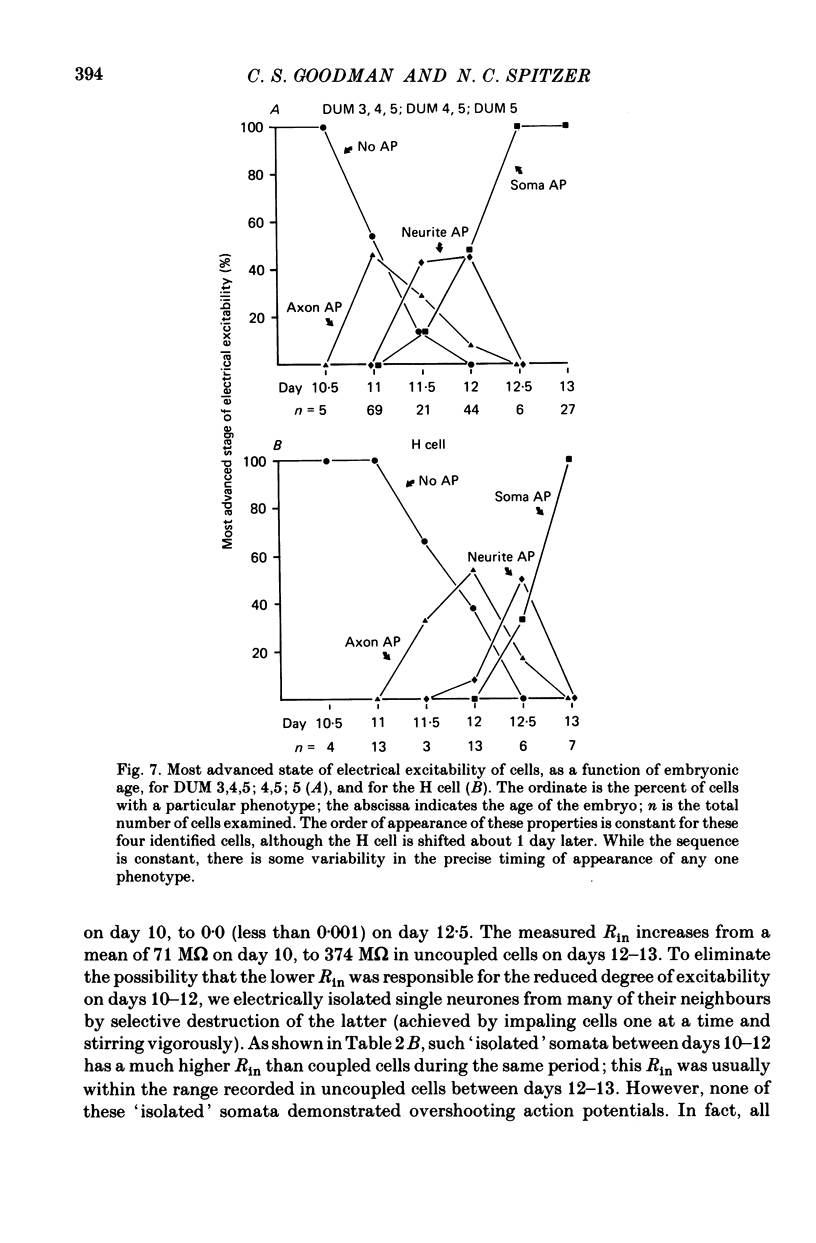
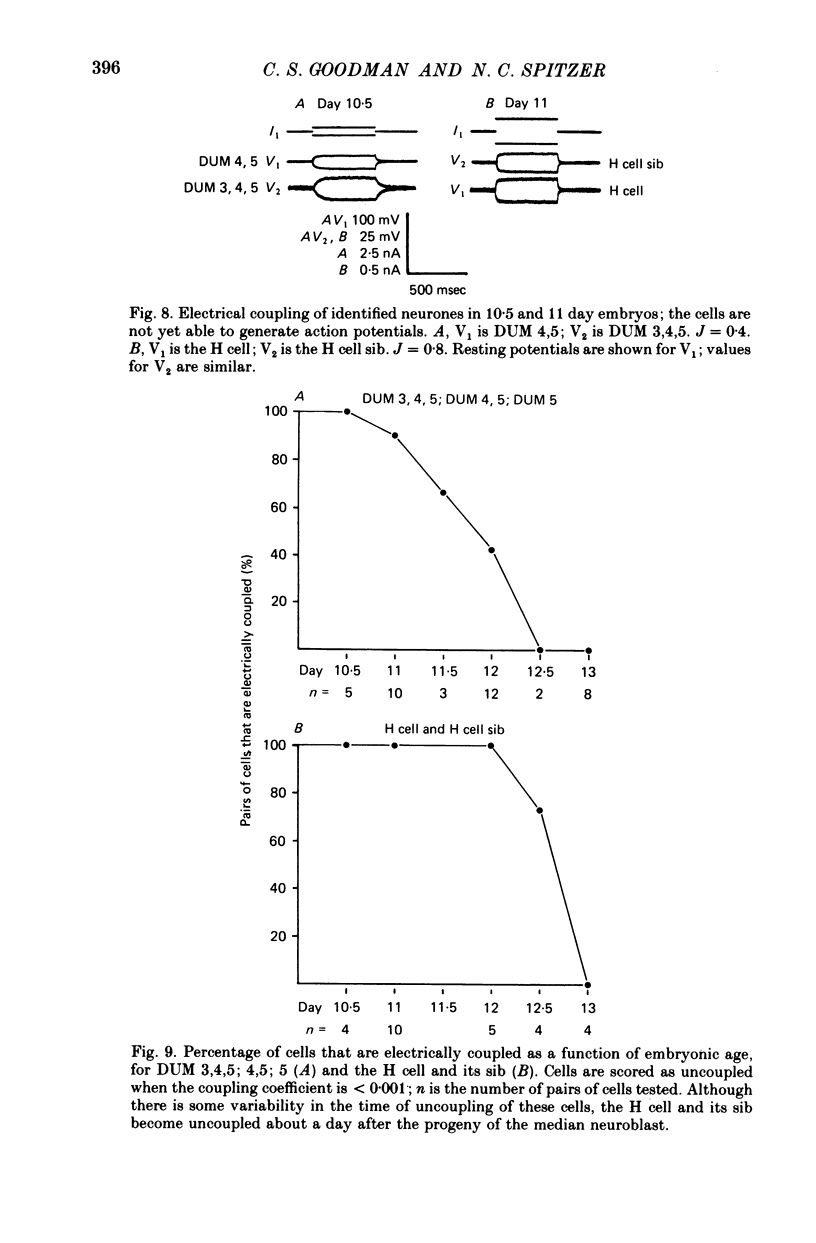
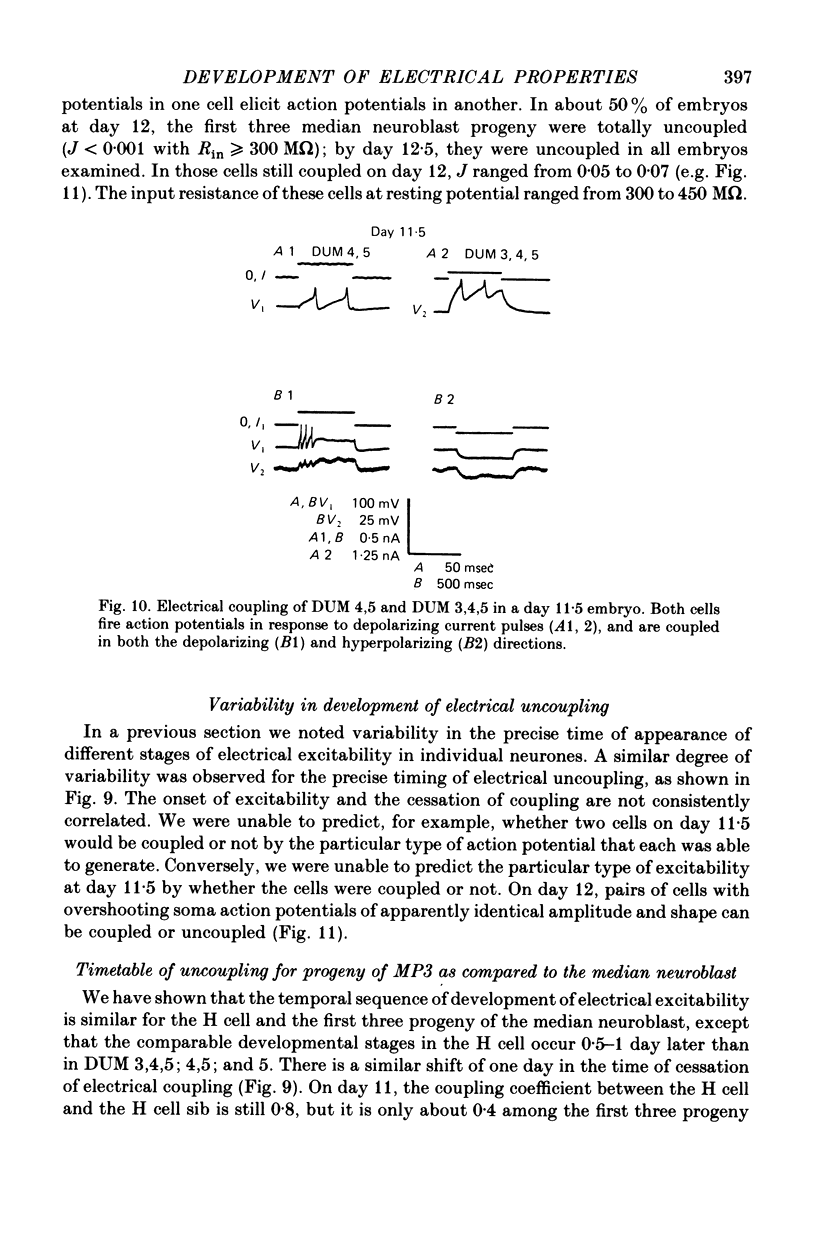
1. We have examined the development of the electrical properties of five identified neurones in grasshopper embryos between days 10 and 13 of embryogenesis (hatching occurs on day 20). DUM 3,4,5; DUM 4,5; DUM 5; the H cell; and the H cell sibling are the progeny of two different precursor cells. Electrical coupling and electrical excitability were assayed by intracellular recordings. 2. Midway through embryogenesis, on day 10, the five cells are highly electrically coupled to each other and are electrically inexcitable. The temporal sequence of the development of electrical excitability and electrical coupling is described for DUM 3,4,5; 4,5; and 5. The H cell and H cell sib undergo the same sequence one day later. 3. The first non-linear membrane property to appear is delayed rectification which appears on day 11 and can be blocked by tetraethylammonium (TEA). In some cells at about day 11, the addition of TEA to normal saline unmasks a Na+-dependent action potential in the axon. 4. The first action potential in normal saline is a Na+-dependent response that appears in the axon at day 11-11.5. 5. The next stage of excitability in normal saline is the appearance about day 11.5 of a Na+-dependent action potential in the median neurite between the soma and the two axons. In some cells at about day 11.5, the addition of TEA unmasks an excitable response in the soma. 6. Overshooting action potentials appear in the soma about day 12; the inward current is carried by both Na+ and Ca2+; TEA causes a prolonged shoulder on the falling phase of the action potential. A short time later, TEA causes a long-duration CA2+ plateau. 7. A progressive decrease in the degree of electrical coupling among the cells occurs between days 10 and 12.5. Complete uncoupling is never observed before day 11, but has always occurred by day 12.5. 8. Two methods were used to demonstrate that electrical coupling does not mask the presence of excitable inward current channels and thus make the cells appear inexcitable. First, we exposed the cells to veratridine. The cells which normally generate excitable Na+ response are depolarized by it; the younger inexcitable cells are not. Secondly, we electrically isolated the cells by killing the somata of their neighbours. The input resistance increased, yet the extent of excitability remained unchanged. 9. There is variability in the precise temporal relationship of excitability and uncoupling. Pairs of cells from different embryos of the same ate can generate the same type of action potentials and yet be coupled in one embryo and uncoupled in another. Electrical excitability and uncoupling appear to be causally unrelated and independent events, occurring at about the same developmental stage.
Full text
PDF

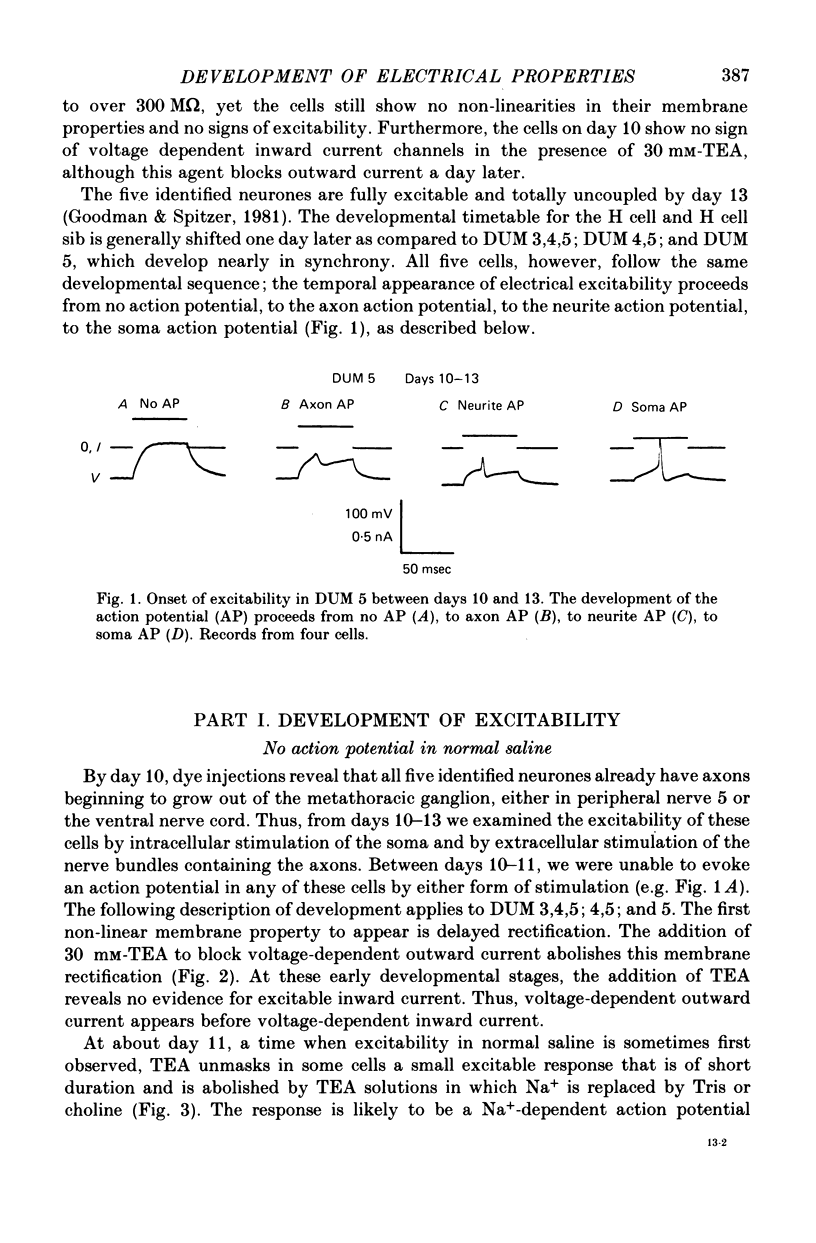
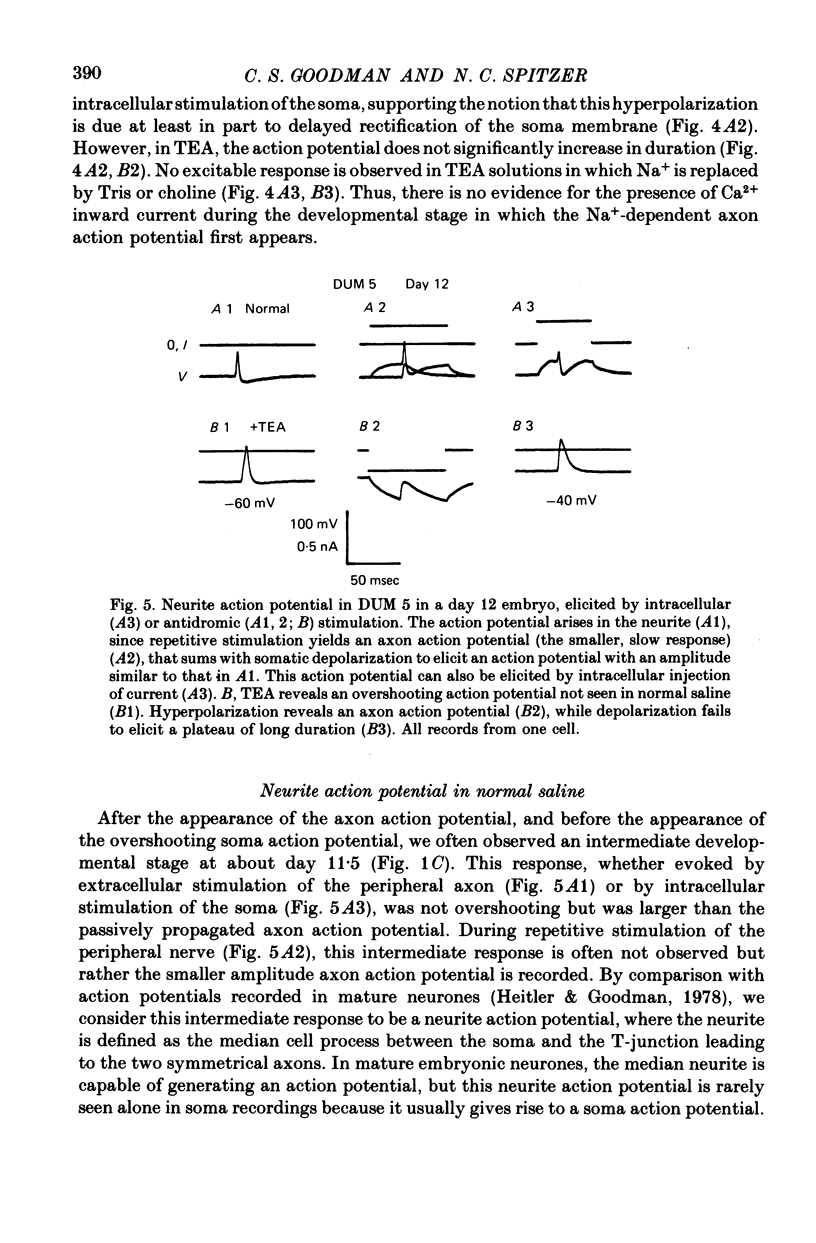







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
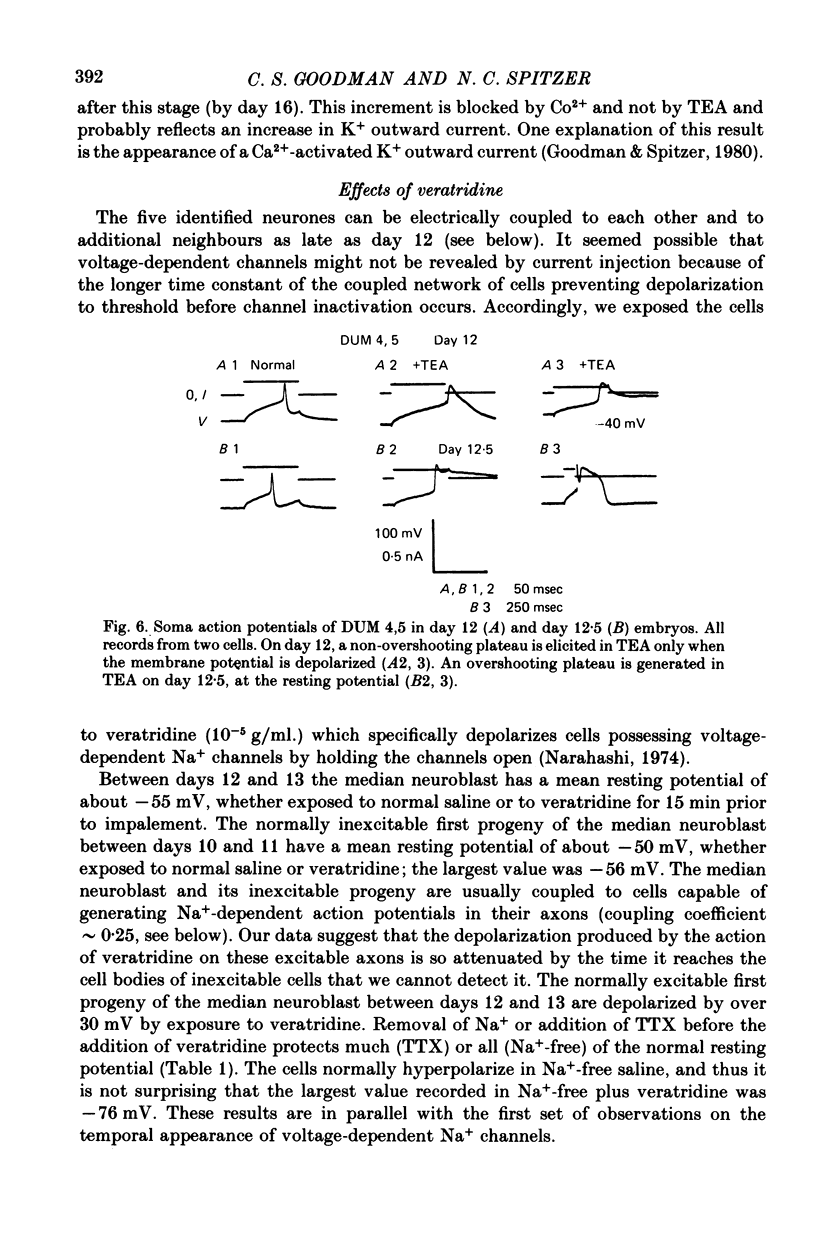
- Baccaglini P. I. Action potentials of embryonic dorsal root ganglion neurones in Xenopus tadpoles. J Physiol. 1978 Oct;283:585–604. doi: 10.1113/jphysiol.1978.sp012521. [DOI] [PMC free article] [PubMed] [Google Scholar]
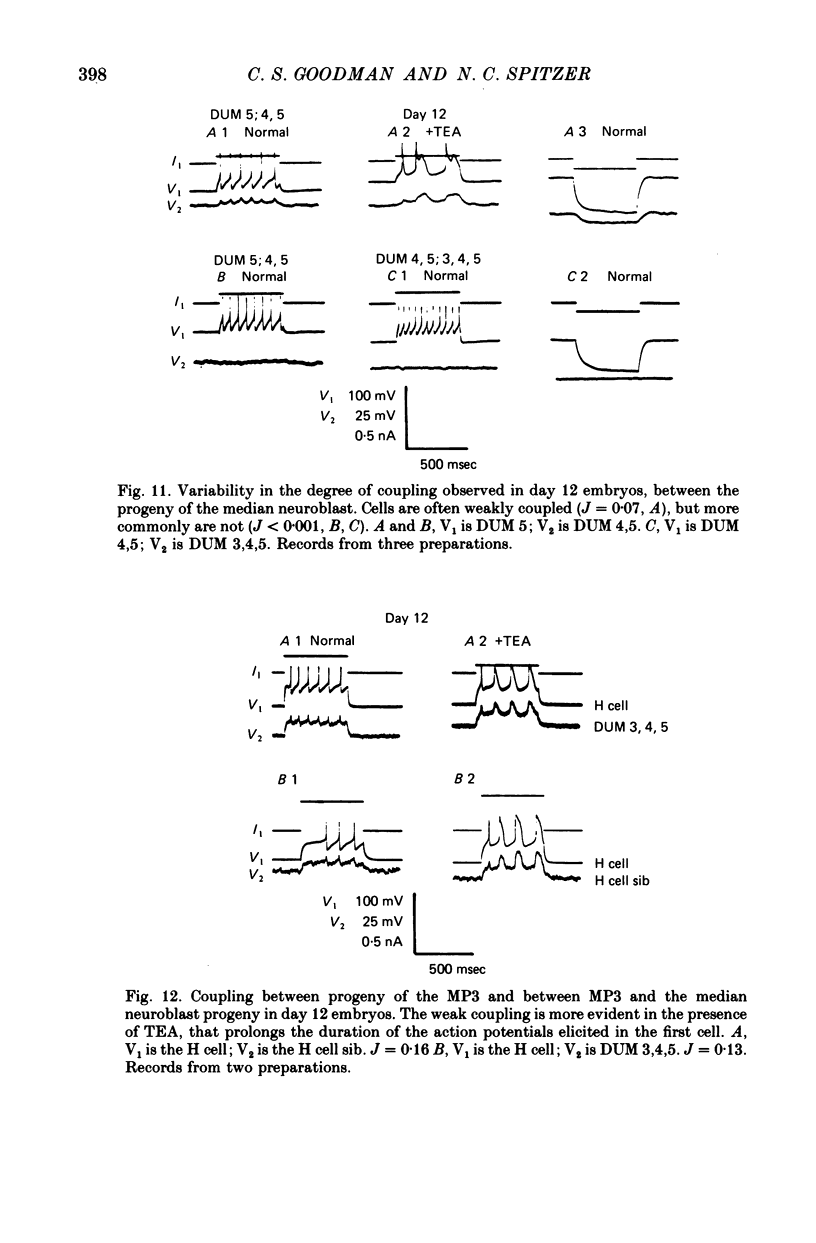
- Baccaglini P. I., Spitzer N. C. Developmental changes in the inward current of the action potential of Rohon-Beard neurones. J Physiol. 1977 Sep;271(1):93–117. doi: 10.1113/jphysiol.1977.sp011992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. S., Spitzer N. C. Embryonic development of identified neurones: differentiation from neuroblast to neurone. Nature. 1979 Jul 19;280(5719):208–214. doi: 10.1038/280208a0. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Spitzer N. C. The mature electrical properties of identified neurones in grasshopper embryos. J Physiol. 1981;313:369–384. doi: 10.1113/jphysiol.1981.sp013671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamborghini J. E. Rohon-beard cells and other large neurons in Xenopus embryos originate during gastrulation. J Comp Neurol. 1980 Jan 15;189(2):323–333. doi: 10.1002/cne.901890208. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Baccaglini P. I. Development of the action potential in embryo amphibian neurons in vivo. Brain Res. 1976 May 14;107(3):610–616. doi: 10.1016/0006-8993(76)90148-7. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Lamborghini J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard A. L. Electrical excitability of outgrowing neurites of embryonic neurones in cultures of dissociated neural plate of Xenopus laevis. J Physiol. 1980 Apr;301:115–128. doi: 10.1113/jphysiol.1980.sp013193. [DOI] [PMC free article] [PubMed] [Google Scholar]


