Abstract
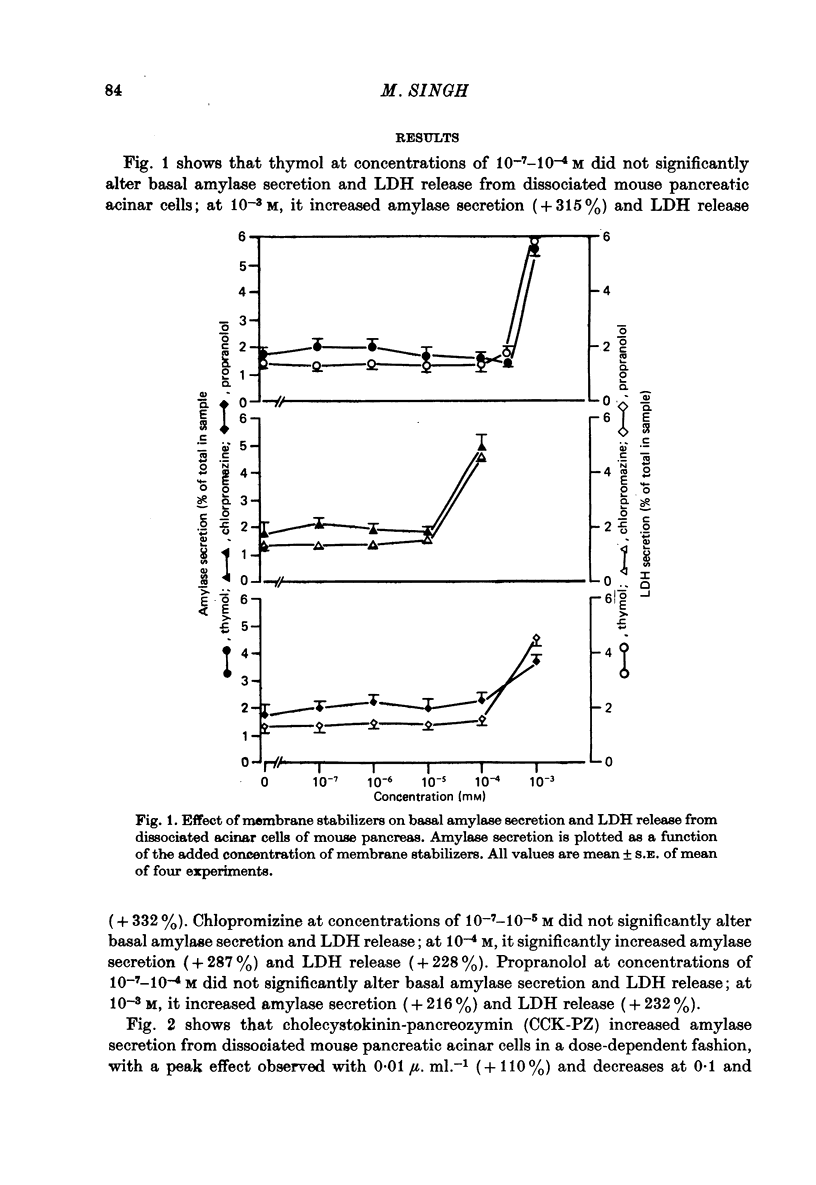
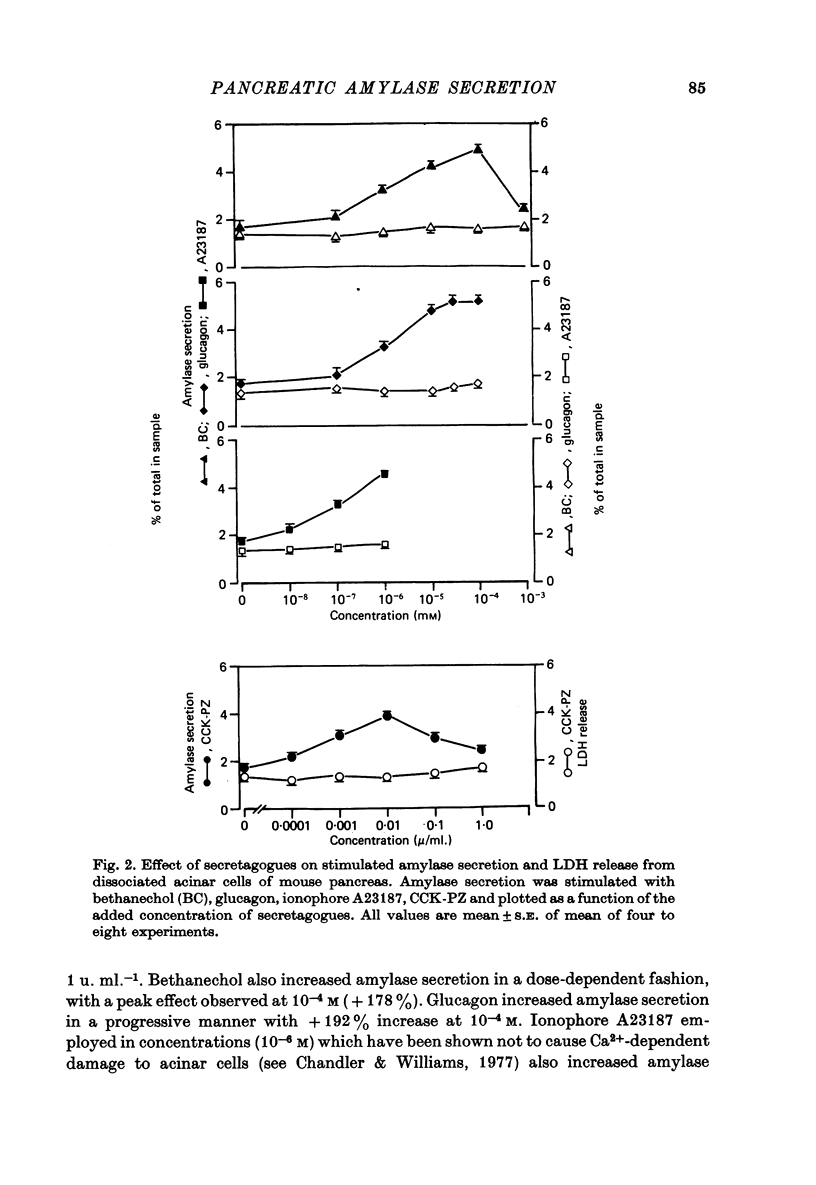
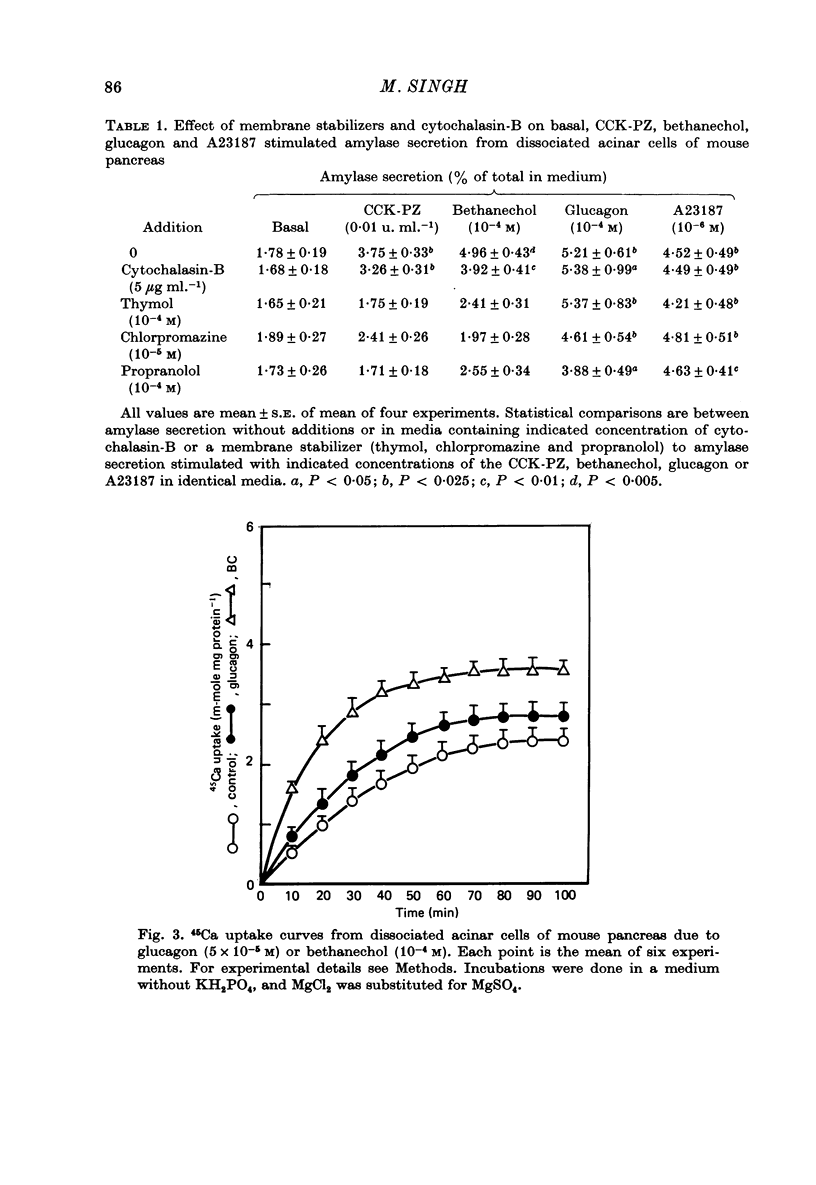
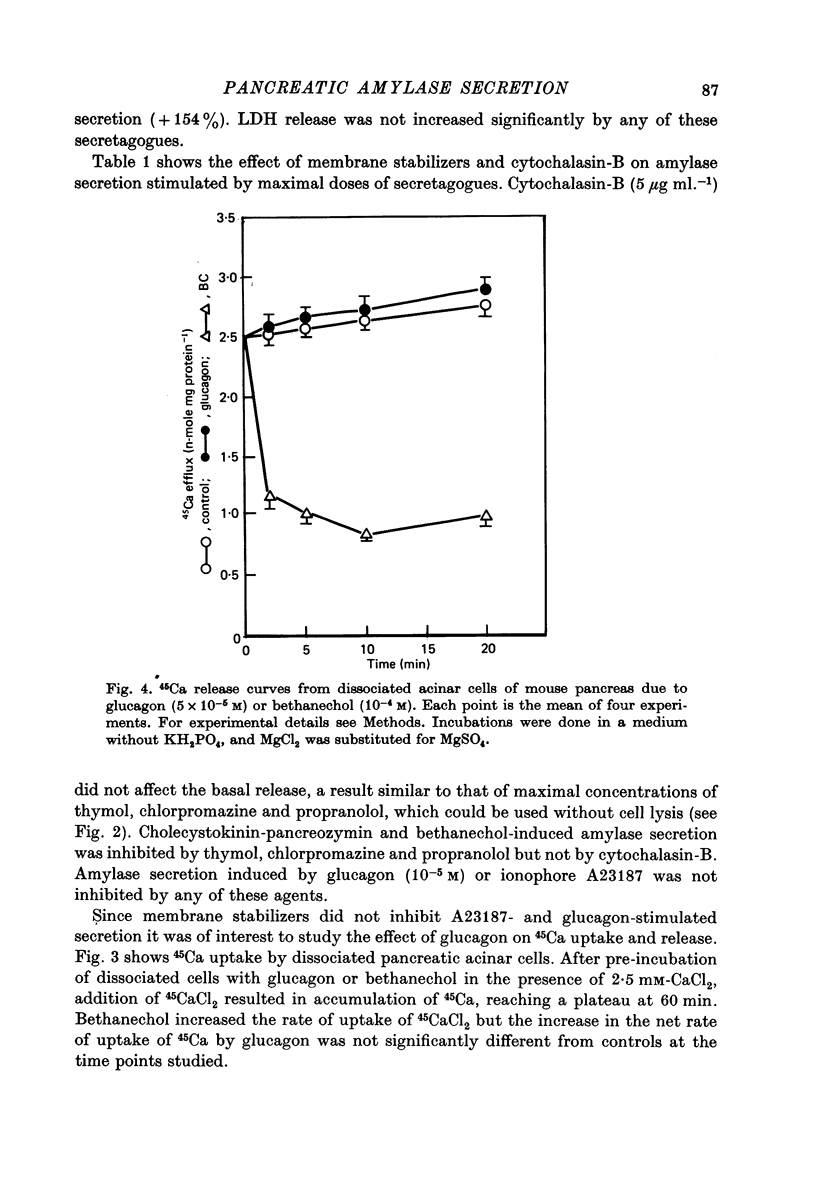
1. The effect of membrane stabilizers and cytochalasin-B on amylase secretion, basal and induced by ionophore A23187, CCK-PZ, bethanechol and glucagon, was studied in dissociated mouse pancreatic acinar cells. 2. Cytochalasin-B did not affect basal or secretagogue-stimulated amylase secretion. 3. Membrane stabilizers [thymol (10(-7)-10(-4) M), chlorpromazine (10(-7)-10(-4) M) and propranolol (10(-7)-10(-5) M) did not alter basal release of amylase. At higher concentrations of thymol (10(-3) M), chlorpromazine (10(-3) M) and propranolol (10(-4) M), dissociated acinar cells were lysed as indicated by an increase in release of lactic dehydrogenase (LDH). 4. Ionophore A23187, CCK-PZ (maximal effective concentrations, 0.01 u. ml.-1), bethanechol (maximal effective concentrations, 10(-4) M) and glucagon increased amylase secretion in a dose-dependent fashion. Concentrations of CCK-PZ and bethanechol beyond optimal levels decreased amylase secretion. Concentrations of ionophore A23187 and glucagon when tested beyond 10(-6) M and 10(-4) M respectively increased the release of LDH. In concentrations that were non-toxic, membrane stabilizers blocked the stimulating effect of cholecystokinin-pancreozymin and bethanechol on amylase secretion but did not alter the response to A23187 and glucagon. 5. Unlike bethanechol, glucagon neither increased the uptake of 45Ca nor did it alter the release of 45Ca from cells previously loaded with 45CaCl2. 6. These data provide evidence that stimulus-secretion coupling in dissociated pancreatic acinar cells is basically similar to cells in situ. The effect of glucagon is consistent with the model in which hormone-dependent mobilization of Ca2+ from intra- or extracellular sources is bypassed leading to digestive enzyme secretion.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1037–1056. doi: 10.1083/jcb.63.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. II. Functional characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1057–1073. doi: 10.1083/jcb.63.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin A. R., Marois C., Dunnigan J., Morisset J. Biochemical reactions involved in pancreatic enzyme secretion. I. Activation of the adenylate cyclase complex. Can J Physiol Pharmacol. 1974 Apr;52(2):174–182. doi: 10.1139/y74-025. [DOI] [PubMed] [Google Scholar]
- Case R. M. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978 May;53(2):211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Intracellular uptake and alpha-amylase and lactate dehydrogenase releasing actions of the divalent cation ionophore A23187 in dissociated pancreatic acinar cells. J Membr Biol. 1977 Apr 22;32(3-4):201–230. doi: 10.1007/BF01905220. [DOI] [PubMed] [Google Scholar]
- Clain J. E., Barbezat G. O., Waterworth M. M., Bank S. Glucagon inhibition of secretin and combined secretin and cholecystokinin stimulated pancreatic exocrine secretion in health and disease. Digestion. 1978;17(1):11–17. doi: 10.1159/000198089. [DOI] [PubMed] [Google Scholar]
- Dyck W. P., Rudick J., Hoexter B., Janowitz H. D. Influence of glucagon on pancreatic exocrine secretion. Gastroenterology. 1969 Mar;56(3):531–537. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Kleveman H. L., Adams T. D., Ondetti M. A. Action of cholecystokinin and cholinergic agents on calcium transport in isolated pancreatic acinar cells. J Clin Invest. 1975 Aug;56(2):366–375. doi: 10.1172/JCI108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenman L. D., Rothman S. S. Diffusion-like process can account for protein secretion by the pancreas. Science. 1979 Jun 15;204(4398):1212–1215. doi: 10.1126/science.451566. [DOI] [PubMed] [Google Scholar]
- Kempen H. J., de Pont J. J., Bonting S. L. Rat pancreas adenylate cyclase V. Its presence in isolated rat pancreatic acinar cells. Biochim Biophys Acta. 1977 Feb 28;496(2):521–531. doi: 10.1016/0304-4165(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Kondo S., Schulz I. Calcium ion uptake in isolated pancreas cells induced by secretagogues. Biochim Biophys Acta. 1976 Jan 8;419(1):76–92. doi: 10.1016/0005-2736(76)90373-4. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Tasler J., Obtulowicz W. Characteristics of inhibition of pancreatic secretion by glucagon. Digestion. 1974;10(2):138–149. doi: 10.1159/000197533. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Tasler J., Obtulowicz W. Effect of glucagon on food-induced gastrointestinal secretions. Digestion. 1973;8(3):220–226. doi: 10.1159/000197317. [DOI] [PubMed] [Google Scholar]
- Lucas M., Schmid G., Kromas R., Löffler G. Calcium metabolism and enzyme secretion in guinea pig pancreas. Uptake, storage and release of calcium in whole cells and mitochondrial and microsomal fractions. Eur J Biochem. 1978 Apr 17;85(2):609–619. doi: 10.1111/j.1432-1033.1978.tb12276.x. [DOI] [PubMed] [Google Scholar]
- Manabe T., Steer M. L. Effects of glucagon on pancreatic content and secretion of amylase in mice. Proc Soc Exp Biol Med. 1979 Sep;161(4):538–542. doi: 10.3181/00379727-161-40592. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Magee D. F. Inhibition of exocrine pancreatic secretion by glucagon and D-glucose given intravenously. Can J Physiol Pharmacol. 1970 May;48(5):299–305. doi: 10.1139/y70-049. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J Physiol. 1975 Jan;244(2):431–465. doi: 10.1113/jphysiol.1975.sp010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renckens B. A., Schrijen J. J., Swarts H. G., de Pont J. J., Bonting S. L. Role of calcium in exocrine pancreatic secretion. IV. Calcium movements in isolated acinar cells of rabbit pancreas. Biochim Biophys Acta. 1978 Dec 1;544(2):338–350. doi: 10.1016/0304-4165(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Shaw H. M., Heath T. J. The effect of glucagon on the formation of pancreatic juice and bile in the rat. Can J Physiol Pharmacol. 1973 Jan;51(1):1–5. doi: 10.1139/y73-001. [DOI] [PubMed] [Google Scholar]
- Singh M., Black O., Webster P. D. Effects of selected drugs on pancreatic macromolecular transport. Gastroenterology. 1973 May;64(5):983–991. [PubMed] [Google Scholar]
- Singh M. Calcium and cyclic nucleotide interaction in secretion of amylase from rat pancreas in vitro. J Physiol. 1979 Nov;296:159–176. doi: 10.1113/jphysiol.1979.sp012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Stimulus-secretion coupling in rat pancreas: role of sodium, calcium and cyclic nucleotides studied by X-537A and BrX-537A. J Physiol. 1980 May;302:1–17. doi: 10.1113/jphysiol.1980.sp013226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A., Cary P., Moffat B. Effects of ions on amylase release by dissociated pancreatic acinar cells. Am J Physiol. 1976 Nov;231(5 Pt 1):1562–1567. doi: 10.1152/ajplegacy.1976.231.5.1562. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Effects of cytochalasin B on pancreatic acinar cell structure and secretion. Cell Tissue Res. 1977 Apr 29;179(4):453–466. doi: 10.1007/BF00219848. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Lee M. Pancreatic acinar cells: use of Ca++ ionophore to separate enzyme release from the earlier steps in stimulus-secretion coupling. Biochem Biophys Res Commun. 1974 Sep 23;60(2):542–548. doi: 10.1016/0006-291x(74)90274-5. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Poulsen J. H., Lee M. Effects of membrane stabilizers on pancreatic amylase release. J Membr Biol. 1977 May 6;33(1-2):185–195. doi: 10.1007/BF01869515. [DOI] [PubMed] [Google Scholar]


