Abstract
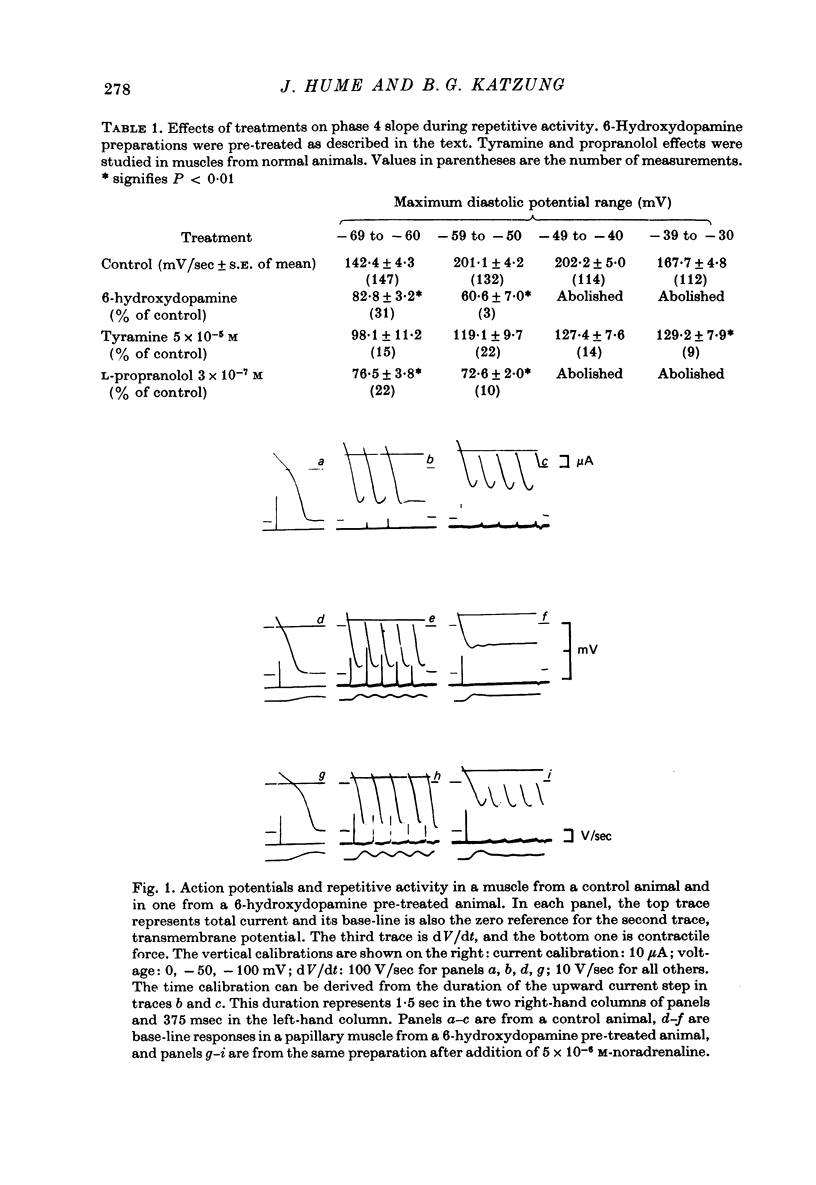
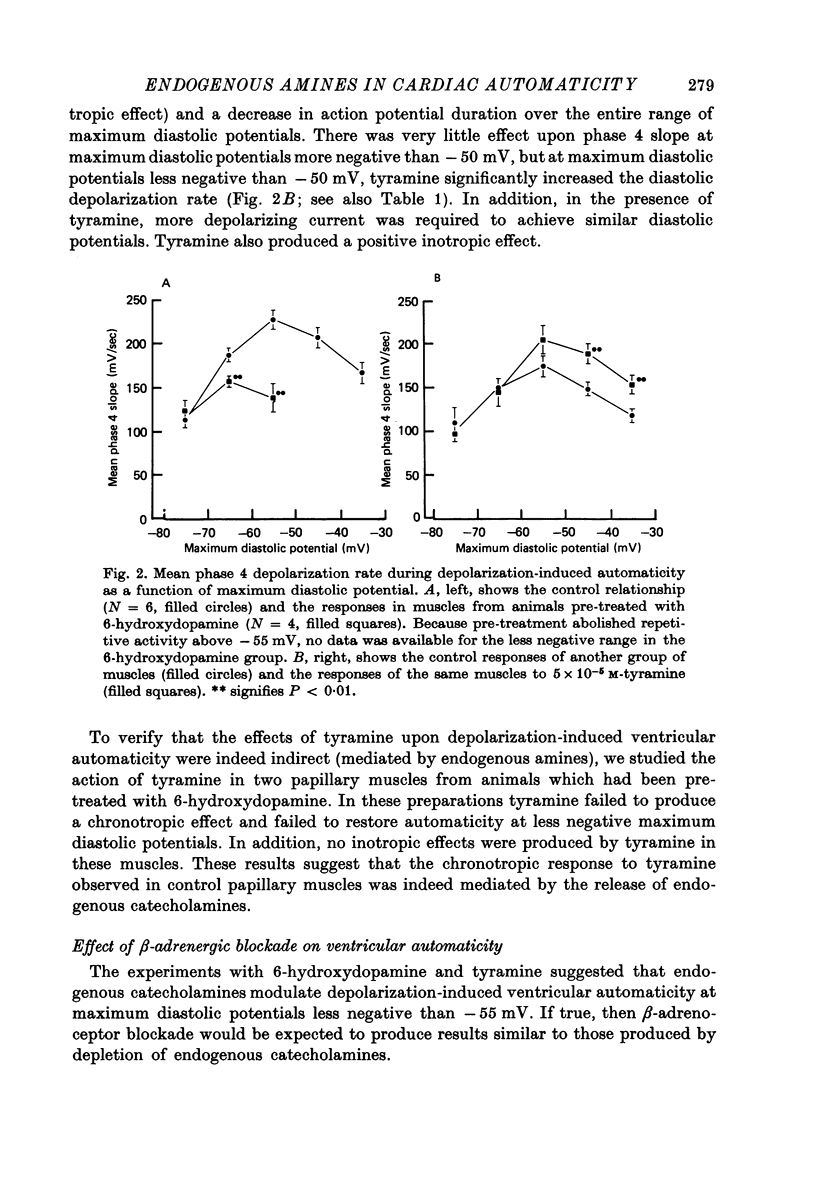
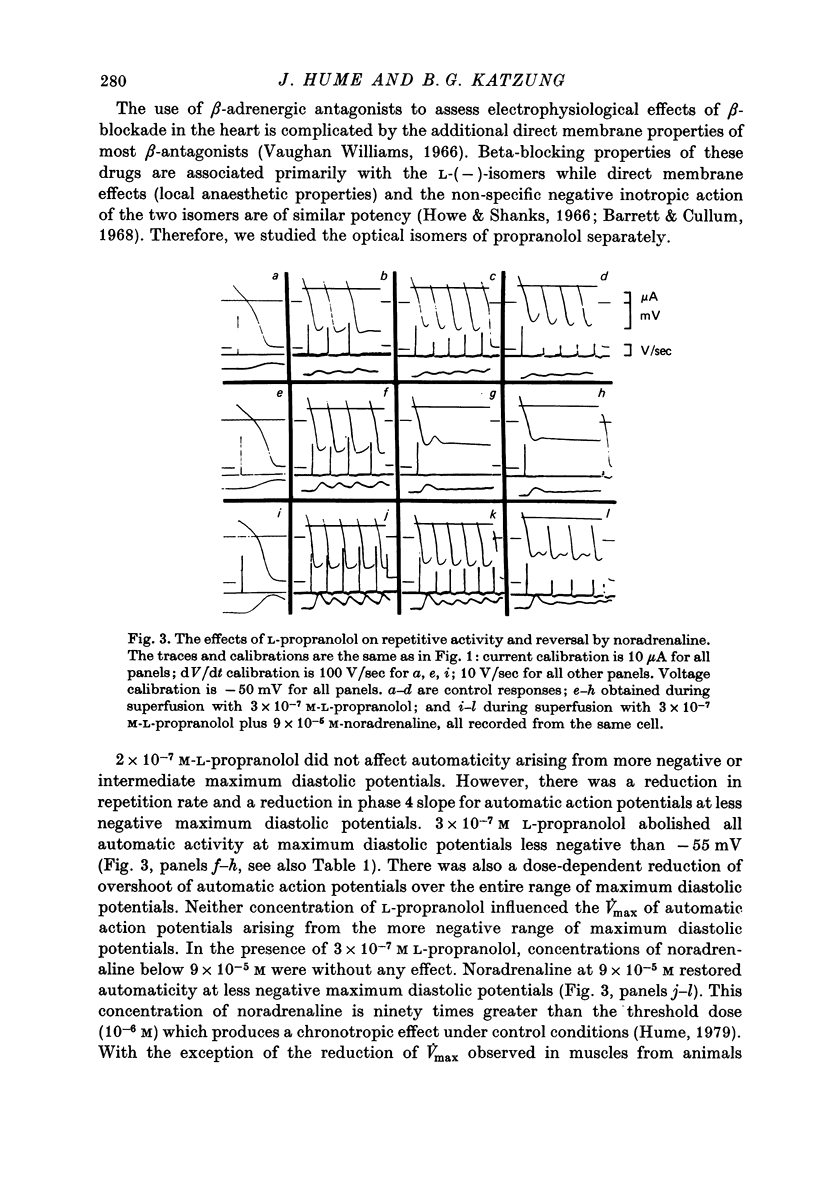
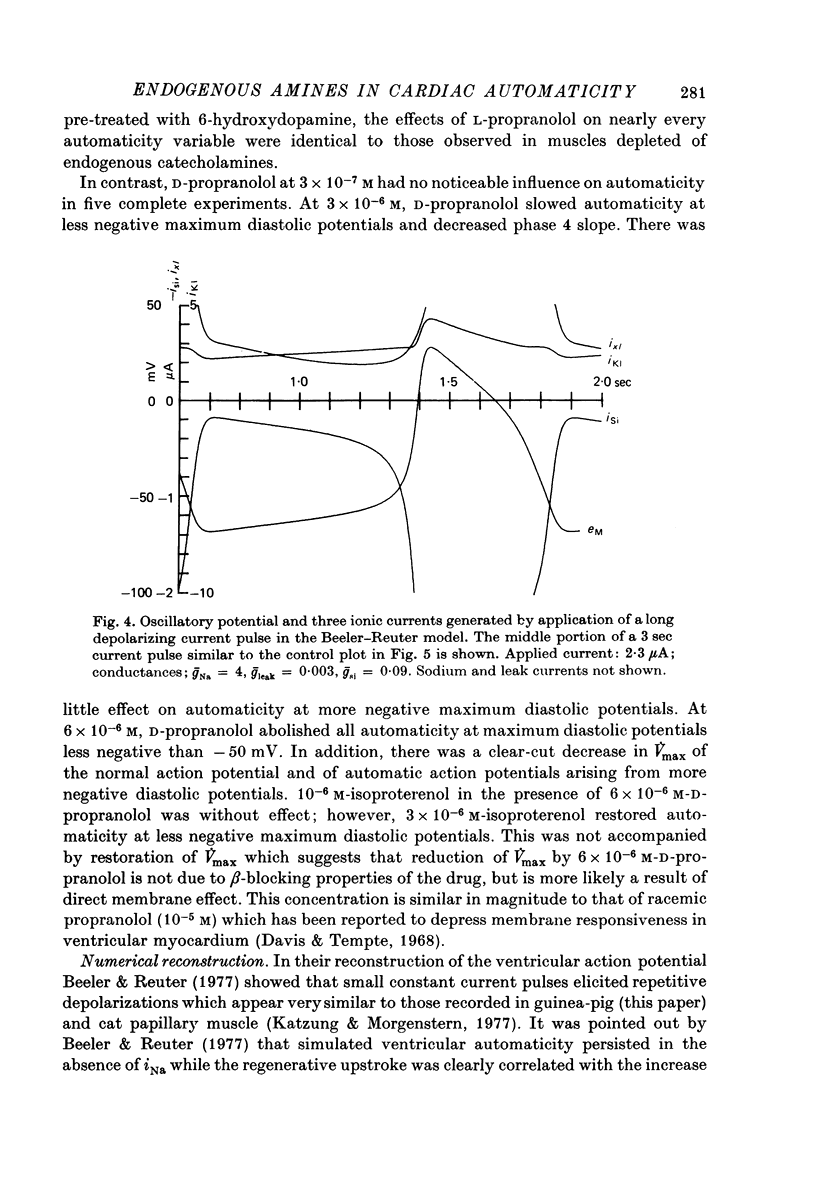
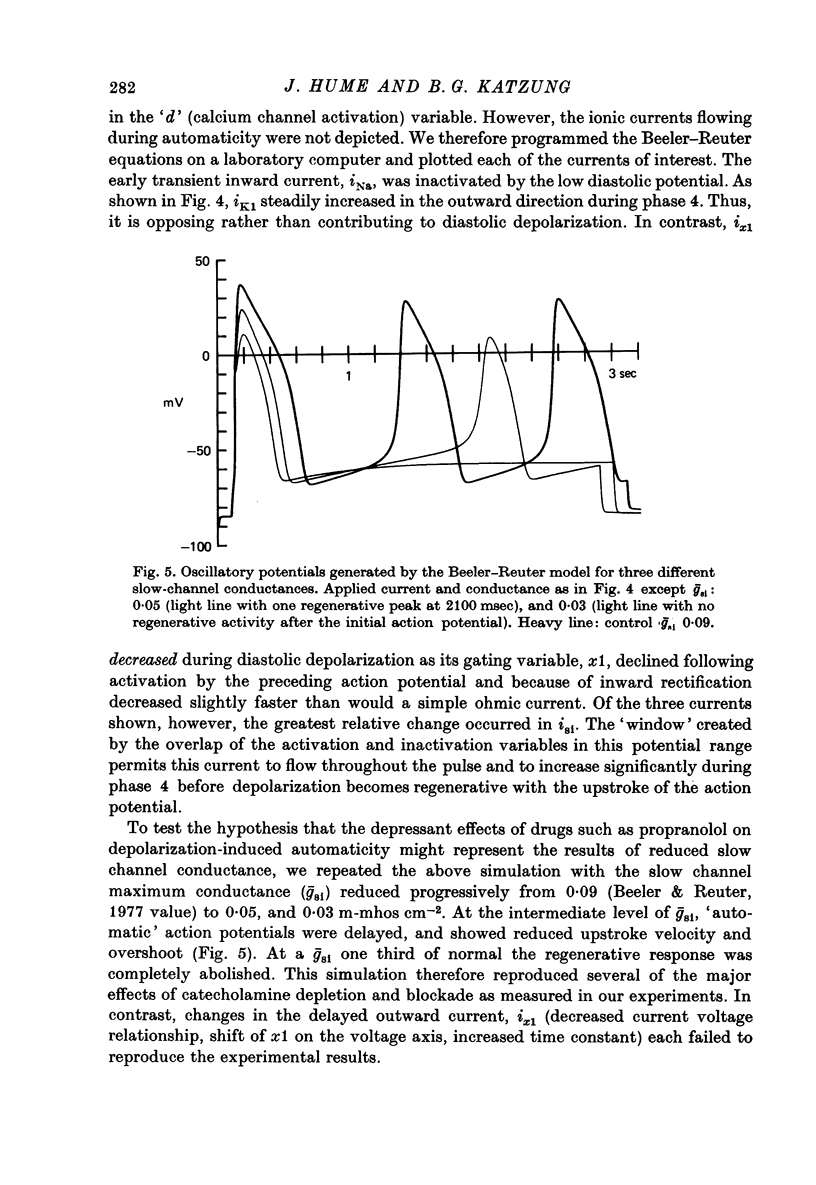
1. Current-clamp experiments were carried out with guinea-pig papillary muscles to determine the dependence of depolarization-induced automaticity on endogenous catecholamines. 2. Catecholamine depletion was produced by pre-treatment of animals with 6-hydroxydopamine and confirmed by fluorimetric assay of right ventricular tissue. Papillary muscles from depleted animals demonstrated a marked suppression of depolarization-induced automaticity for maximum diastolic potentials less negative than -55 mV. This suppression was completely reversed by noradrenaline but not by tyramine. 3. In normal tissue, noradrenaline and tyramine had much smaller effects on automaticity arising from maximum diastolic potentials negative to -55 mV than on repetitive activity arising positive to this level. 4. L-propranolol in concentrations of 2-3 x 10(-7) M reduced repetitive activity in the less negative range of maximum diastolic potential. No evidence of direct membrane depression was observed at these doses and the effect was reversed by application of noradrenaline. 5. D-propranolol, the isomer with much lower beta-receptor blocking potency, required twentyfold higher concentrations to depress automaticity and this was accompanied by evidence of direct membrane depression, i.e. reduction of upstroke velocity of action potentials. 6. These results show that automaticity induced in guinea-pig papillary muscles by depolarization positive to -55 mV is strongly dependent upon endogenous catecholamines. 7. The hypothesis that endogenous catecholamines facilitate depolarization-induced automaticity through an increase in calcium conductance was modelled using numerical techniques. It was found that changes in calcium conductance caused changes in the model which closely parallelled the experimental effects of catecholamine depletion and beta-blockade. The effects of changes in delayed rectification in the model did not accurately reproduce the experimental results.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMORY D. W., WEST T. C. Chronotropic response following direct electrical stimulation of the isolated sinoatrial node: a pharmacologic evaluation. J Pharmacol Exp Ther. 1962 Jul;137:14–23. [PubMed] [Google Scholar]
- Arita M., Saikawa T., Nagamoto Y. Spontaneous electrical activity induced by depolarizing currents in canine ventricular myocardium. A preliminary note. Jpn Heart J. 1976 Mar;17(2):246–257. doi: 10.1536/ihj.17.246. [DOI] [PubMed] [Google Scholar]
- Ariëns E. J. The structure-activity relationships of beta adrenergic drugs and beta adrenergic blocking drugs. Ann N Y Acad Sci. 1967 Feb 10;139(3):606–631. doi: 10.1111/j.1749-6632.1967.tb41232.x. [DOI] [PubMed] [Google Scholar]
- Arnsdorf M. F. The effect of antiarrhythmic drugs on triggered sustained rhythmic activity in cardiac Purkinje fibers. J Pharmacol Exp Ther. 1977 Jun;201(3):689–700. [PubMed] [Google Scholar]
- Barrett A. M., Cullum V. A. The biological properties of the optical isomers of propranolol and their effects on cardiac arrhythmias. Br J Pharmacol. 1968 Sep;34(1):43–55. doi: 10.1111/j.1476-5381.1968.tb07949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R. Field stimulation as a means of effecting the graded release of autonomic transmitters in isolated heart muscle. J Pharmacol Exp Ther. 1966 Feb;151(2):221–235. [PubMed] [Google Scholar]
- Brennan F. J., Cranefield P. F., Wit A. L. Effects of lidocaine and on slow response and depressed fast response action potentials of canine cardiac Purkinje fibers. J Pharmacol Exp Ther. 1978 Feb;204(2):312–324. [PubMed] [Google Scholar]
- Brown H. F., Giles W., Noble S. J. Membrane currents underlying activity in frog sinus venosus. J Physiol. 1977 Oct;271(3):783–816. doi: 10.1113/jphysiol.1977.sp012026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Noble S. J. Membrane currents underlying delayed rectification and pace-maker activity in frog atrial muscle. J Physiol. 1969 Oct;204(3):717–736. doi: 10.1113/jphysiol.1969.sp008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H., Difrancesco D., Noble S. Cardiac pacemaker oscillation and its modulation by autonomic transmitters. J Exp Biol. 1979 Aug;81:175–204. doi: 10.1242/jeb.81.1.175. [DOI] [PubMed] [Google Scholar]
- CROUT J. R., MUSKUS A. J., TRENDELENBURG U. Effect of tyramine on isolated guinea-pig atria in relation to their noradrenaline stores. Br J Pharmacol Chemother. 1962 Jun;18:600–611. doi: 10.1111/j.1476-5381.1962.tb01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. D., Temte J. V. Effects of propranolol on the transmembrane potentials of ventricular muscle and Purkinje fibers of the dog. Circ Res. 1968 May;22(5):661–677. doi: 10.1161/01.res.22.5.661. [DOI] [PubMed] [Google Scholar]
- Endoh M., Hashimoto K. Pharmacological evidence of autonomic nerve activities in canine papillary muscle. Am J Physiol. 1970 May;218(5):1459–1463. doi: 10.1152/ajplegacy.1970.218.5.1459. [DOI] [PubMed] [Google Scholar]
- Foster M, Dew-Smith A G. The effects of the Constant Current on the Heart. J Anat Physiol. 1876 Jul;10(Pt 4):735–771. [PMC free article] [PubMed] [Google Scholar]
- Grant A. O., Katzung B. G. The effects of quinidine and verapamil on electrically induced automaticity in the ventricular myocardium of guinea pig. J Pharmacol Exp Ther. 1976 Feb;196(2):407–419. [PubMed] [Google Scholar]
- Hashimoto K., Hauswirth O., Wehner H. D., Ziskoven R. The relation between the current underlying pacemaker activity and beta-adrenoceptors in cardiac Purkinje fibres: a study using adrenaline, procaine, atenolol and penbutolol. Naunyn Schmiedebergs Arch Pharmacol. 1979 May;307(1):9–19. doi: 10.1007/BF00506546. [DOI] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. The mechanism of oscillatory activity at low membrane potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):255–265. doi: 10.1113/jphysiol.1969.sp008691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe R., Shanks R. G. Optical isomers of propranolol. Nature. 1966 Jun 25;210(5043):1336–1338. doi: 10.1038/2101336a0. [DOI] [PubMed] [Google Scholar]
- Imanishi S. Calcium-sensitive discharges in canine Purkinje fibers. Jpn J Physiol. 1971 Aug;21(4):443–463. doi: 10.2170/jjphysiol.21.443. [DOI] [PubMed] [Google Scholar]
- Imanishi S., McAllister R. G., Jr, Surawicz B. The effects of verapamil and lidocaine on the automatic depolarizations in guinea-pig ventricular myocardium. J Pharmacol Exp Ther. 1978 Nov;207(2):294–303. [PubMed] [Google Scholar]
- Imanishi S., Surawicz B. Automatic activity in depolarized guinea pig ventricular myocardium. Characteristics and mechanisms. Circ Res. 1976 Dec;39(6):751–759. doi: 10.1161/01.res.39.6.751. [DOI] [PubMed] [Google Scholar]
- Josephson I., Sperelakis N. Local anesthetic blockade of Ca2+ -mediated action potentials in cardiac muscle. Eur J Pharmacol. 1976 Dec;40(2):201–208. doi: 10.1016/0014-2999(76)90053-4. [DOI] [PubMed] [Google Scholar]
- Katzung B. G. Effects of extracellular calcium and sodium on depolarization-induced automaticity in guinea pig papillary muscle. Circ Res. 1975 Jul;37(1):118–127. doi: 10.1161/01.res.37.1.118. [DOI] [PubMed] [Google Scholar]
- Katzung B. G., Morgenstern J. A. Effects of extracellular potassium on ventricular automaticity and evidence for a pacemaker current in mammalian ventricular myocardium. Circ Res. 1977 Jan;40(1):105–111. doi: 10.1161/01.res.40.1.105. [DOI] [PubMed] [Google Scholar]
- Katzung B. Electrically induced automaticity in ventricular myocardium. Life Sci. 1974 Mar 16;14(6):1133–1140. doi: 10.1016/0024-3205(74)90237-9. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D., Tsien R. W. Reconstruction of the electrical activity of cardiac Purkinje fibres. J Physiol. 1975 Sep;251(1):1–59. doi: 10.1113/jphysiol.1975.sp011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaman M. W., Weinreich D., McCaman R. E. The determination of picomole levels of 5-hydroxytryptamine and dopamine in Aplysia, Tritonia and leech nervous tissues. Brain Res. 1973 Apr 13;53(1):129–137. doi: 10.1016/0006-8993(73)90772-5. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. Uber den Einfluss der extracellulären Ca-Konzentration auf Membranpotential und Kontraktion isolierter Herzpräparate bei graduierter Depolarisation. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;300(2):87–107. [PubMed] [Google Scholar]
- SPERELAKIS N., LEHMKUHL D. EFFECT OF CURRENT ON TRANSMEMBRANE POTENTIALS IN CULTURED CHICK HEART CELLS. J Gen Physiol. 1964 May;47:895–927. doi: 10.1085/jgp.47.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Tranzer J. P. The pharmacology of 6-hydroxydopamine. Annu Rev Pharmacol. 1973;13:169–180. doi: 10.1146/annurev.pa.13.040173.001125. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Effects of epinephrine on the pacemaker potassium current of cardiac Purkinje fibers. J Gen Physiol. 1974 Sep;64(3):293–319. doi: 10.1085/jgp.64.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. M. Mode of action of beta receptor antagonists on cardiac muscle. Am J Cardiol. 1966 Sep;18(3):399–405. doi: 10.1016/0002-9149(66)90062-2. [DOI] [PubMed] [Google Scholar]


