Abstract
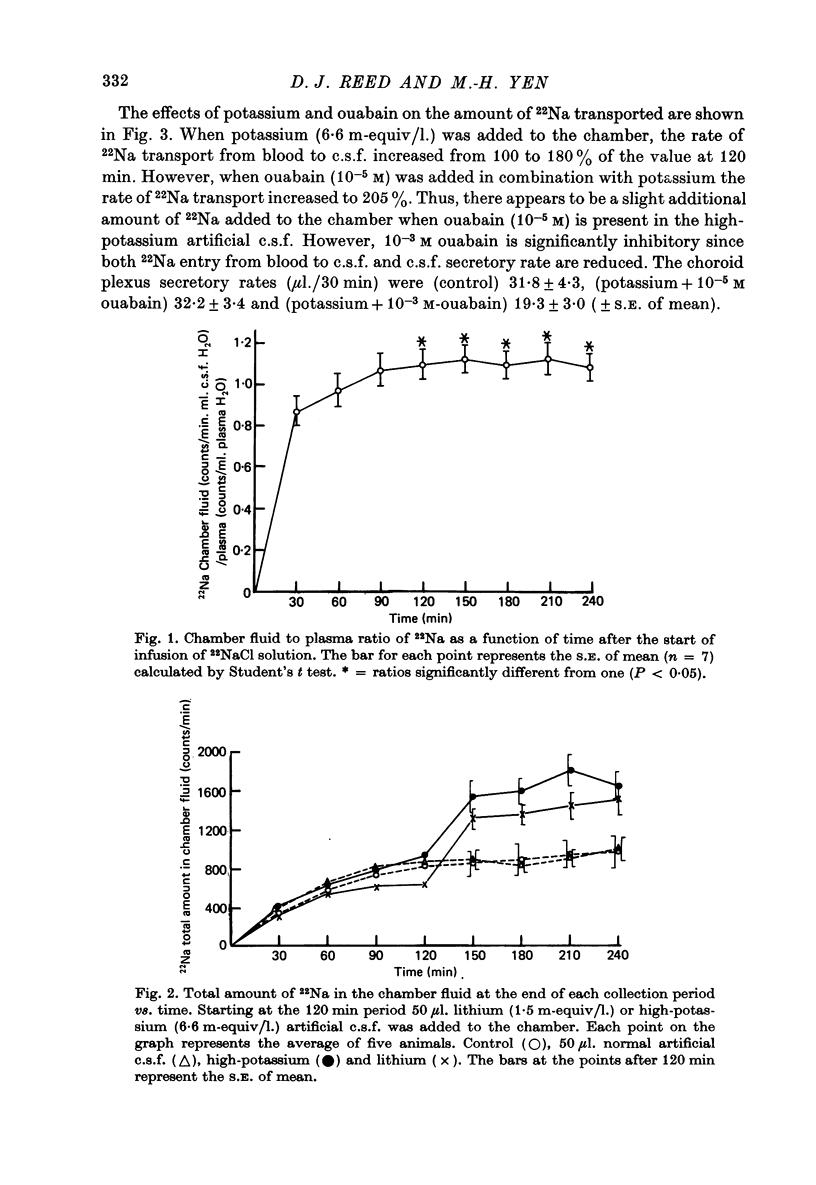
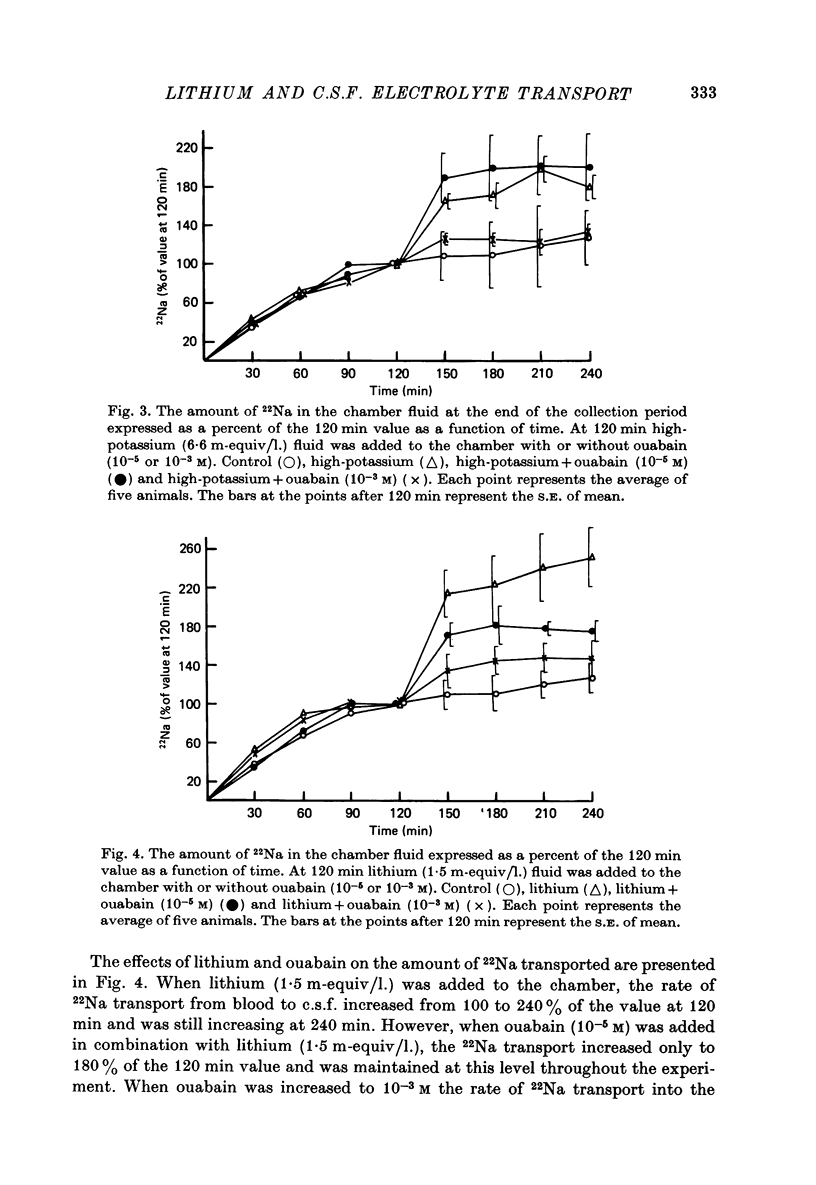
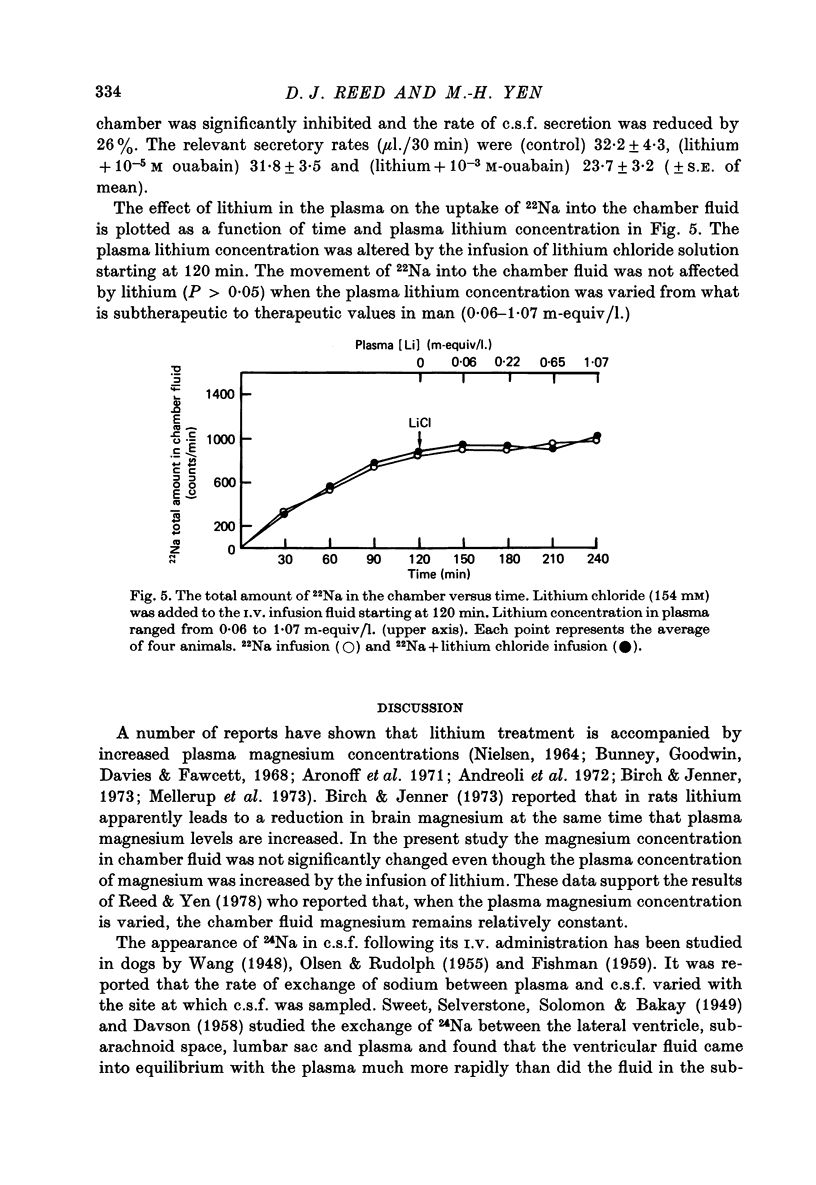
1. The effects of lithium on electrolyte transport were studied by using the cat choroid plexus isolated in a chamber in situ. 2. Lithium infused intravenously to produce plasma lithium concentrations up to 5 m-equiv/l. caused an increase in plasma magnesium with no effect on the concentration of magnesium in the chamber fluid. 3. When 22NaCl was infused intravenously the chamber fluid/plasma ratio of 22Na was nearly 1 in the first 30 min sample and at the steady state it was significantly greater than 1. 4. When lithium chloride (1.5 m-equiv/l.) or potassium chloride (6.6 m-equiv/l.) was added to the chamber at the start of a collection period with plasma 22Na in the steady state, the 22Na content of the chamber fluid promptly increased 118 and 68%, respectively, above the control value with no increase in secretory rate. 5. The addition of ouabain to the chamber fluid, in addition to the lithium chloride or potassium chloride, tended to stimulate or have no significant effect on 22Na uptake at a concentration of 10(-5) M and to reduce it as well as the secretory rate at 10(-3) M. 6. The date are compatible with there being two functionally separate sodium transport systems in the choroid plexus. One transports sodium accompanied by an anion and water to provide the fluid secreted into the chamber (c.s.f.) and the other operates primarily to regulate the potassium concentration of the c.s.f. by pumping potassium out in exchange for sodium. 7. Lithium can be transported by both systems to a limited extent and the presence of lithium in the c.s.f. stimulates the sodium-potassium regulating pump.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES A., 3rd, SAKANOUE M., ENDO S. NA, K, CA, MG, AND C1 CONCENTRATIONS IN CHOROID PLEXUS FLUID AND CISTERNAL FLUID COMPARED WITH PLASMA ULTRAFILTRATE. J Neurophysiol. 1964 Jul;27:672–681. doi: 10.1152/jn.1964.27.4.672. [DOI] [PubMed] [Google Scholar]
- Akera T., Brody T. M., So R. H., Tobin T., Baskin S. I. Factors and agents that influence cardiac glycoside-Na+, K+-ATPase interaction. Ann N Y Acad Sci. 1974;242(0):617–634. doi: 10.1111/j.1749-6632.1974.tb19121.x. [DOI] [PubMed] [Google Scholar]
- Andreoli V. M., Villani F., Brambilla G. Increased calcium and magnesium excretion induced by lithium carbonate. Psychopharmacologia. 1972;25(1):77–85. doi: 10.1007/BF00422619. [DOI] [PubMed] [Google Scholar]
- Aronoff M. S., Evens R. G., Durell J. Effect of lithium salts on electrolyte metabolism. J Psychiatr Res. 1971 Jun;8(2):139–159. doi: 10.1016/0022-3956(71)90015-x. [DOI] [PubMed] [Google Scholar]
- Baer L., Kassir S., Fieve R. Lithium-induced changes in electrolyte balance and tissue electrolyte concentration. Psychopharmacologia. 1970;17(3):216–224. doi: 10.1007/BF00402081. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Ortiz O. Lithium-stimulated sodium efflux in frog skeletal muscle. Biochim Biophys Acta. 1970 Dec 1;219(2):479–483. doi: 10.1016/0005-2736(70)90226-9. [DOI] [PubMed] [Google Scholar]
- Birch N. J., Jenner F. A. The distribution of lithium and its effects on the distribution and excretion of other ions in the rat. Br J Pharmacol. 1973 Mar;47(3):586–594. doi: 10.1111/j.1476-5381.1973.tb08189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanski D. F., Brodie B. B. Role of sodium and potassium ions in storage of norepinephrine by sympathetic nerve endings. Life Sci. 1966 Sep;5(17):1563–1569. doi: 10.1016/0024-3205(66)91025-3. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Stulcová B. Efflux mechanism contributing to the stability of the potassium concentration in cerebrospinal fluid. J Physiol. 1970 Jun;208(2):415–430. doi: 10.1113/jphysiol.1970.sp009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney W. E., Jr, Goodwin F. K., Davis J. M., Fawcett J. A. A behavioral-biochemical study of lithium treatment. Am J Psychiatry. 1968 Oct;125(4):499–512. doi: 10.1176/ajp.125.4.499. [DOI] [PubMed] [Google Scholar]
- COPPEN A., SHAW D. M. MINERAL METABOLISM IN MELANCHOLIA. Br Med J. 1963 Dec 7;2(5370):1439–1444. doi: 10.1136/bmj.2.5370.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. Potassium exchange between cerebrospinal fluid, plasma, and brain. Am J Physiol. 1965 Dec;209(6):1219–1226. doi: 10.1152/ajplegacy.1965.209.6.1219. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. THE MECHANISM OF ISOTONIC WATER TRANSPORT. J Gen Physiol. 1964 Sep;48:15–42. doi: 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Functional consequences of ultrastructural geometry in "backwards" fluid-transporting epithelia. J Cell Biol. 1968 Jun;37(3):694–702. doi: 10.1083/jcb.37.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN R. A. Factors influencing the exchange of sodium between plasma and cerebrospinal fluid. J Clin Invest. 1959 Oct;38:1698–1708. doi: 10.1172/JCI103948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS J. L. Total body sodium and potassium in depressive illness. Clin Sci. 1960 Feb;19:133–138. [PubMed] [Google Scholar]
- Glen A. I., Bradbury M. W., Wilson J. Stimulation of the sodium pump in the red blood cell by lithium and potassium. Nature. 1972 Oct 13;239(5372):399–401. doi: 10.1038/239399a0. [DOI] [PubMed] [Google Scholar]
- Goldman R. H., Coltart D. J., Friedman J. P., Nola G. T., Berke D. K., Schweizer E., Harrison D. C. The inotropic effects of digoxin in hyperkalemia. Relation to (Na+,K+)-ATPase inhibition in the intact animal. Circulation. 1973 Oct;48(4):830–838. doi: 10.1161/01.cir.48.4.830. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E. Effects of potassium and lithium on sodium transport from blood to cerebrospinal fluid. J Neurochem. 1977 Mar;28(3):597–603. doi: 10.1111/j.1471-4159.1977.tb10431.x. [DOI] [PubMed] [Google Scholar]
- Ho A. K., Gershon S., Pinckney L. The effects of acute and prolonged lithium treatment on the distribution of electrolytes, potassium and sodium. Arch Int Pharmacodyn Ther. 1970 Jul;186(1):54–65. [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Horst W. D., Kopin I. J., Ramey E. R. Influence of sodium and calcium on norepinephrine uptake by isolated perfused rat hearts. Am J Physiol. 1968 Oct;215(4):817–822. doi: 10.1152/ajplegacy.1968.215.4.817. [DOI] [PubMed] [Google Scholar]
- Hullin R. P., Swinscoe J. C., McDonald R., Dransfield G. A. Metabolic balance studies on the effect of lithium salts in manic-depressive psychosis. Br J Psychiatry. 1968 Dec;114(517):1561–1573. doi: 10.1192/bjp.114.517.1561. [DOI] [PubMed] [Google Scholar]
- Husted R. F., Reed D. J. Regulation of cerebrospinal fluid potassium by the cat choroid plexus. J Physiol. 1976 Jul;259(1):213–221. doi: 10.1113/jphysiol.1976.sp011462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C. E., Reed D. J., Woodbury D. M. Active transport of sodium and potassium by the choroid plexus of the rat. J Physiol. 1974 Sep;241(2):359–372. doi: 10.1113/jphysiol.1974.sp010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaghey P. D., Maizels M. Cation exchanges of lactose-treated human red cells. J Physiol. 1962 Aug;162(3):485–509. doi: 10.1113/jphysiol.1962.sp006946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellerup E. T., Plenge P., Rafaelsen O. J. Lithium effects on magnesium metabolism in rats. Int Pharmacopsychiatry. 1973;8(3):178–183. doi: 10.1159/000467989. [DOI] [PubMed] [Google Scholar]
- Miner L. C., Reed D. J. Composition of fluid obtained from choroid plexus tissue isolated in a chamber in situ. J Physiol. 1972 Dec;227(1):127–139. doi: 10.1113/jphysiol.1972.sp010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIELSEN J. MAGNESIUM--LITHIUM STUDIES 1. SERUM AND ERYTHROCYTE MAGNESIUM IN PATIENTS WITH MANIC STATES DURING LITHIUM TREATMENT. Acta Psychiatr Scand. 1964;40:190–196. doi: 10.1111/j.1600-0447.1964.tb05745.x. [DOI] [PubMed] [Google Scholar]
- OLSEN N. S., RUDOLPH G. G. Transfer of sodium and bromide ions between blood, cerebrospinal fluid and brain tissue. Am J Physiol. 1955 Dec;183(3):427–432. doi: 10.1152/ajplegacy.1955.183.3.427. [DOI] [PubMed] [Google Scholar]
- Pesce M. A., Strande C. S. A new micromethod for determination of protein in cerebrospinal fluid and urine. Clin Chem. 1973 Nov;19(11):1265–1267. [PubMed] [Google Scholar]
- Pollay M. Formation of cerebrospinal fluid. Relation of studies of isolated choroid plexus to the standing gradient hypothesis. J Neurosurg. 1975 Jun;42(6):665–673. doi: 10.3171/jns.1975.42.6.0665. [DOI] [PubMed] [Google Scholar]
- Quinton P. M., Wright E. M., Tormey J. M. Localization of sodium pumps in the choroid plexus epithelium. J Cell Biol. 1973 Sep;58(3):724–730. doi: 10.1083/jcb.58.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed D. J., Yen M. H. The role of the cat choroid plexus in regulating cerebrospinal fluid magnesium. J Physiol. 1978 Aug;281:477–485. doi: 10.1113/jphysiol.1978.sp012434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. B., Pollay M. The secretion of cerebrospinal fluid. Exp Eye Res. 1977;25 (Suppl):127–148. doi: 10.1016/s0014-4835(77)80012-2. [DOI] [PubMed] [Google Scholar]
- Sjodin R. A., Beaugé L. A. Strophanthidin-sensitive components of potassium and sodium movements in skeletal muscle as influenced by the internal sodium concentration. J Gen Physiol. 1968 Sep;52(3):389–407. doi: 10.1085/jgp.52.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Balagura S. The effect of lithium chloride on the electrolyte composition of cerebrospinal fluid of the rat. Physiol Behav. 1972 Aug;9(2):261–262. doi: 10.1016/0031-9384(72)90246-6. [DOI] [PubMed] [Google Scholar]
- VATES T. S., Jr, BONTING S. L., OPPELT W. W. NA-K ACTIVATED ADENOSINE TRIPHOSPHATASE FORMATION OF CEREBROSPINAL FLUID IN THE CAT. Am J Physiol. 1964 May;206:1165–1172. doi: 10.1152/ajplegacy.1964.206.5.1165. [DOI] [PubMed] [Google Scholar]
- WANG J. C. Penetration of radioactive sodium and chloride into cerebrospinal fluid and aqueous humor. J Gen Physiol. 1948 Jan 30;31(3):259–268. doi: 10.1085/jgp.31.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELCH K. SECRETION OF CEREBROSPINAL FLUID BY CHOROID PLEXUS OF THE RABBIT. Am J Physiol. 1963 Sep;205:617–624. doi: 10.1152/ajplegacy.1963.205.3.617. [DOI] [PubMed] [Google Scholar]
- Whittembury G., Proverbio F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970;316(1):1–25. doi: 10.1007/BF00587893. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Mechanisms of ion transport across the choroid plexus. J Physiol. 1972 Oct;226(2):545–571. doi: 10.1113/jphysiol.1972.sp009997. [DOI] [PMC free article] [PubMed] [Google Scholar]


