Abstract
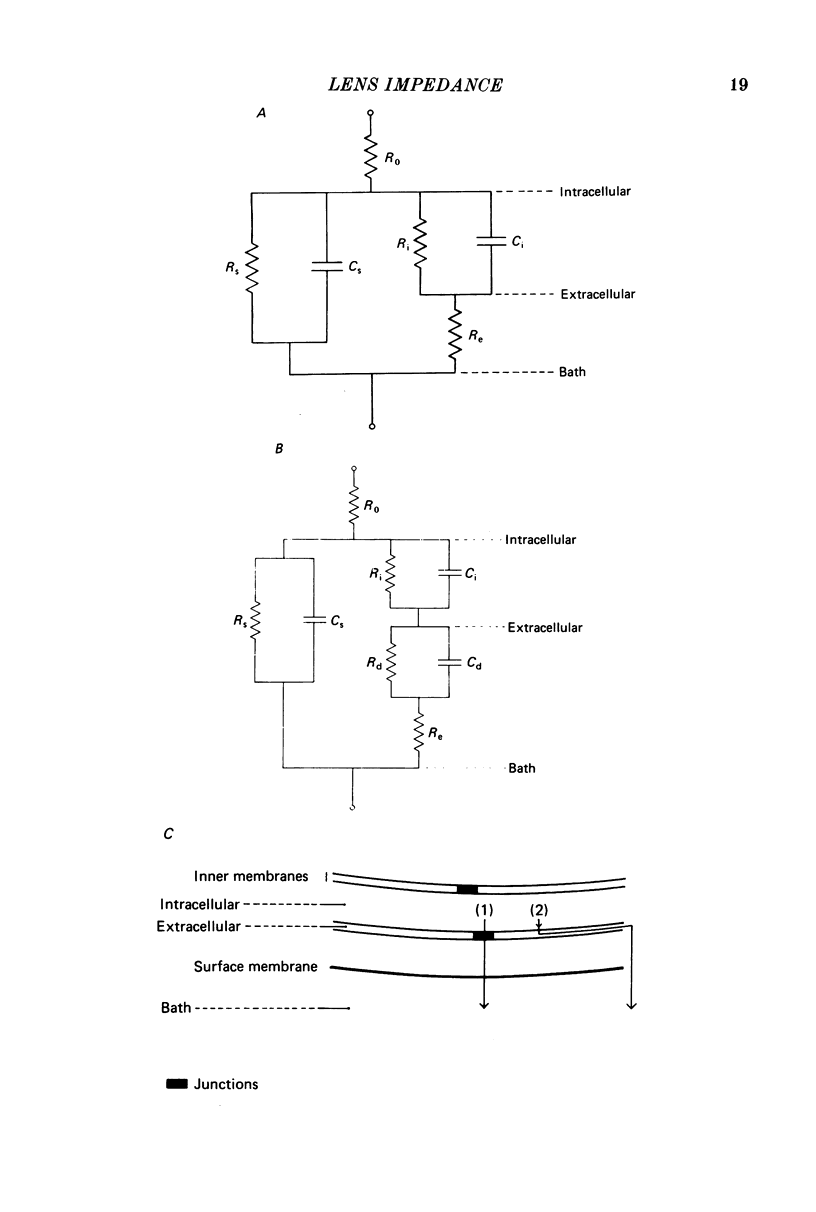
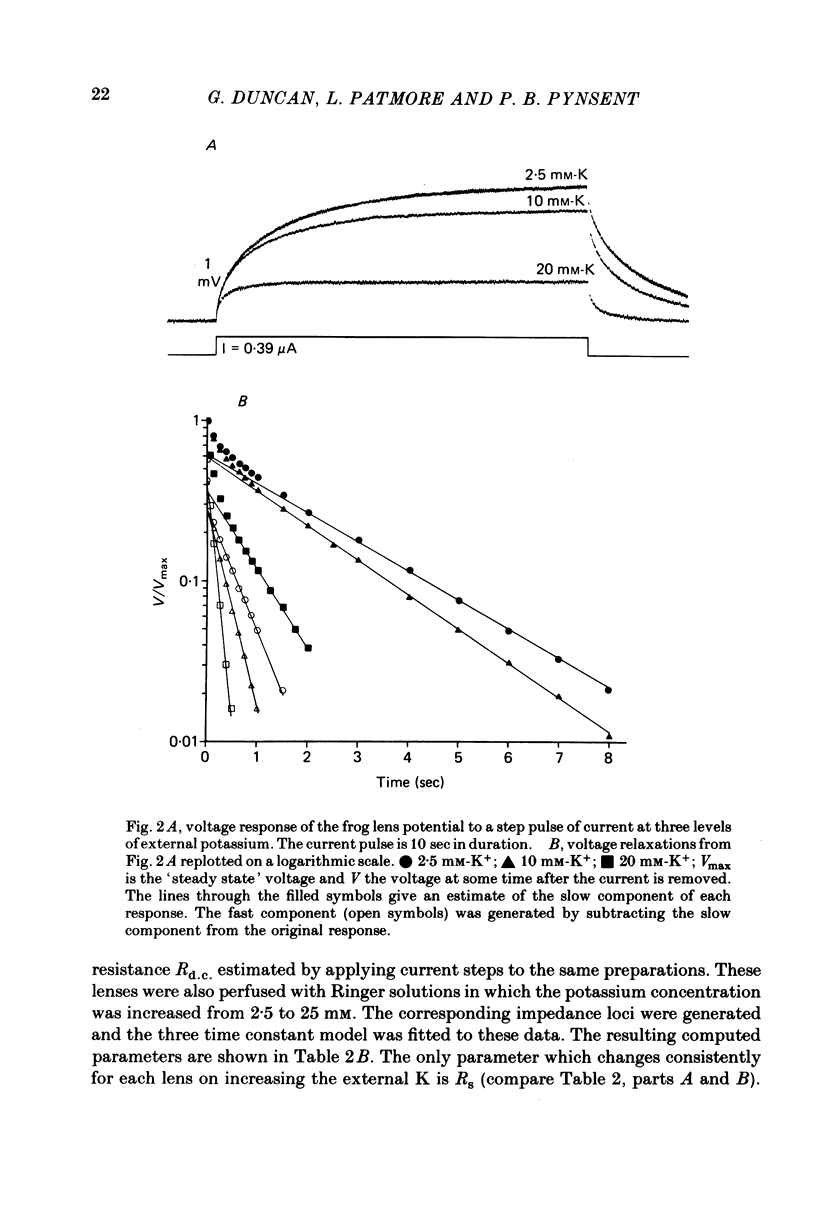
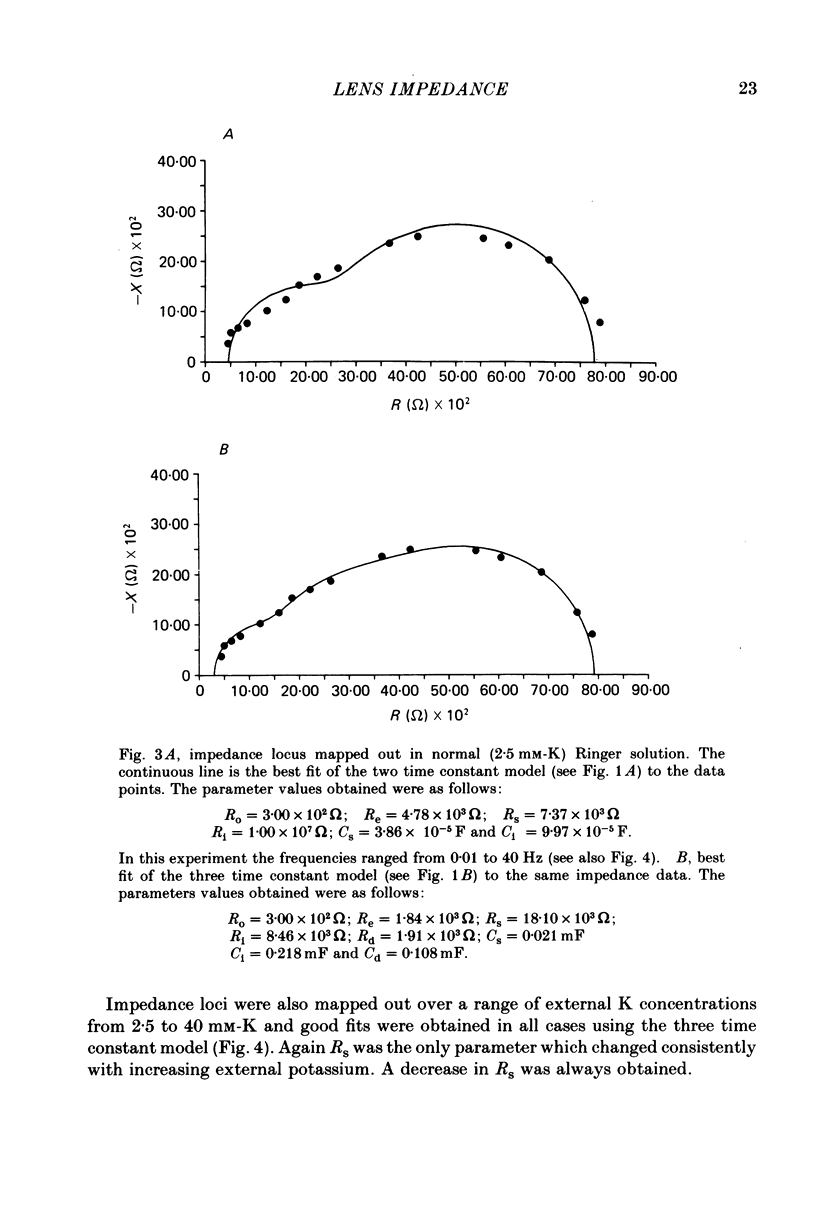
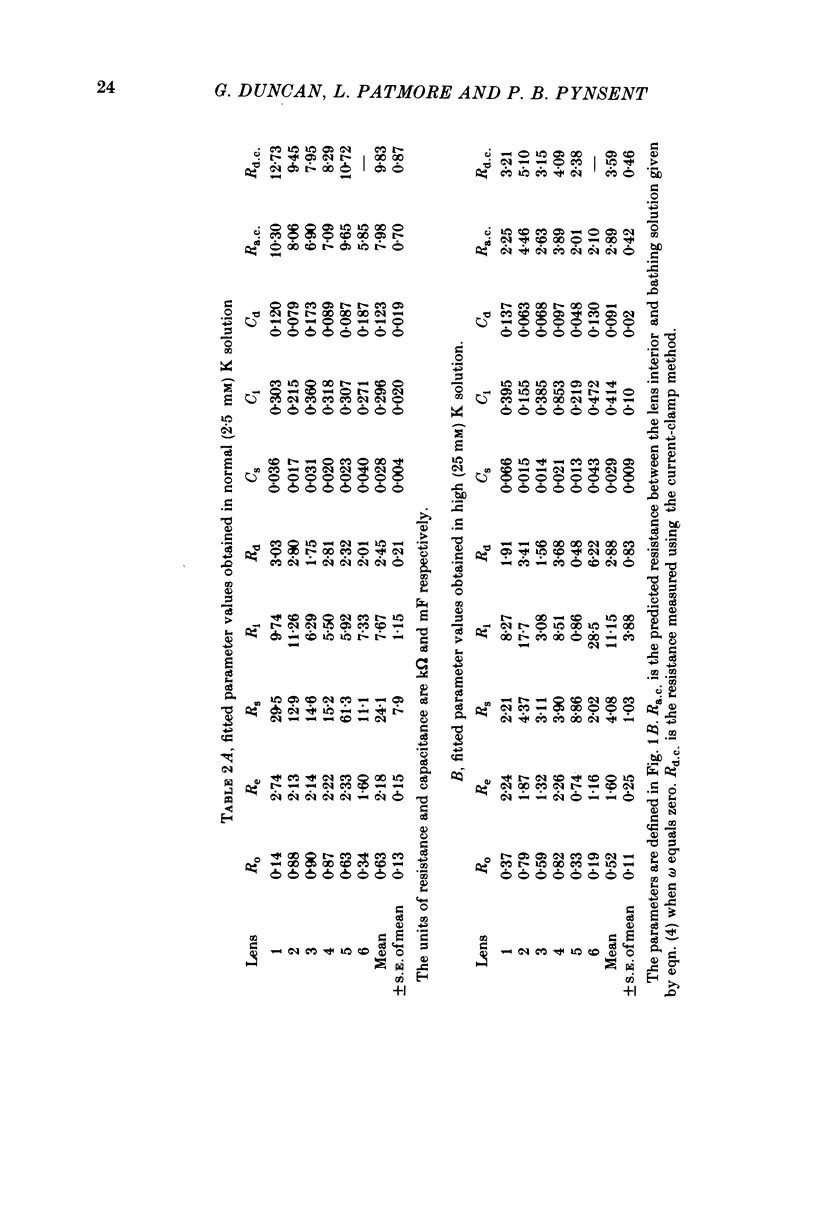
1. The electrical resistance of the perfused frog lens was measured using separate internal current passing and voltage measuring electrodes. 2. The resistance values obtained using voltage clamp and direct and alternating current techniques were in good agreement. 3. The voltage transients induced in response to current steps were multi-exponential in form. Increasing the external K concentration reduced both the amplitude of the voltage response and the rise time. 4. The impedance characteristics were investigated in more detail using alternating current analysis techniques. 5. In an equivalent-circuit modelling study it was assumed that there were two major pathways for current flow in the lens. The first through the surface membranes and the second through the inner fibre membranes via the narrow extracellular spaces. 6. The experimental impedance loci could not be adequately fitted by a simple two time constant model and a third time constant was introduced which may represent diffusion polarization effects in the extracellular spaces. 7. The three time constant model gave good and consistent fits to impedance data from a number of preparations. 8. The form of the impedance loci was also dependent on the external K concentration, but the only fitted parameter which changed consistently with external K was the surface membrane resistance (Rs).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN A. I. THE ELECTRON MICROSCOPY OF THE NORMAL HUMAN LENS. Invest Ophthalmol. 1965 Aug;4:433–446. [PubMed] [Google Scholar]
- Caspar D. L., Goodenough D. A., Makowski L., Phillips W. C. Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J Cell Biol. 1977 Aug;74(2):605–628. doi: 10.1083/jcb.74.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen C., Lewis S. A., Diamond J. M. Impedance analysis of a tight epithelium using a distributed resistance model. Biophys J. 1979 May;26(2):291–317. doi: 10.1016/S0006-3495(79)85250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamere N. A., Duncan G. A comparison of ion concentrations, potentials and conductances of amphibian, bovine and cephalopod lenses. J Physiol. 1977 Oct;272(1):167–186. doi: 10.1113/jphysiol.1977.sp012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamere N. A., Paterson C. A. The influence of calcium-free EGTA solution upon membrane permeability in the crystalline lens of the frog. J Gen Physiol. 1978 May;71(5):581–593. doi: 10.1085/jgp.71.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamere N. A., Paterson C. A. The influence of calcium-free solutions upon permeability characteristics of the rabbit lens. Exp Eye Res. 1979 Jan;28(1):45–53. doi: 10.1016/0014-4835(79)90104-0. [DOI] [PubMed] [Google Scholar]
- Duncan G. The site of the ion restricting membranes in the toad lens. Exp Eye Res. 1969 Oct;8(4):406–412. doi: 10.1016/s0014-4835(69)80006-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Rae J. L. Current-voltage relationships in the crystalline lens. J Physiol. 1976 Nov;262(2):285–300. doi: 10.1113/jphysiol.1976.sp011596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- Kidder G. W., 3rd, Rehm W. S. A model for the long time-constant transient voltage response to current in epithelial tissues. Biophys J. 1970 Mar;10(3):215–236. doi: 10.1016/S0006-3495(70)86295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R. T., Rae J. L., Eisenberg R. S. Electrical properties of structural components of the crystalline lens. Biophys J. 1979 Jan;25(1):181–201. doi: 10.1016/S0006-3495(79)85284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes D. H., Rehm W. S. Unstirred-layer model for the long time-constant transient voltage response to current in epithelial tissue. J Theor Biol. 1971 Jul;32(1):25–45. doi: 10.1016/0022-5193(71)90133-0. [DOI] [PubMed] [Google Scholar]
- Patmore L., Duncan G. A TEA-sensitive component in the conductance of a non-excitable tissue (the amphibian lens). Exp Eye Res. 1979 Mar;28(3):349–352. doi: 10.1016/0014-4835(79)90096-4. [DOI] [PubMed] [Google Scholar]
- Rae J. L., Blankenship J. E. Bioelectric measurements in the frog lens. Exp Eye Res. 1973 Feb;15(2):209–217. doi: 10.1016/0014-4835(73)90121-8. [DOI] [PubMed] [Google Scholar]
- Rae J. L. The movement of procion dye in the crystalline lens. Invest Ophthalmol. 1974 Feb;13(2):147–150. [PubMed] [Google Scholar]
- Rehm W. S., Shoemaker R. L., Sanders S. S., Tarvin J. T., Wright J. A., Jr, Friday E. A. Conductance of epithelial tissues with particular reference to the frog's cornea and gastric mucosa. Exp Eye Res. 1973 May 10;15(5):533–552. doi: 10.1016/0014-4835(73)90066-3. [DOI] [PubMed] [Google Scholar]
- Smith P. G. The low-frequency electrical impedance of the isolated frog skin. Acta Physiol Scand. 1971 Mar;81(3):355–366. doi: 10.1111/j.1748-1716.1971.tb04910.x. [DOI] [PubMed] [Google Scholar]


