Abstract
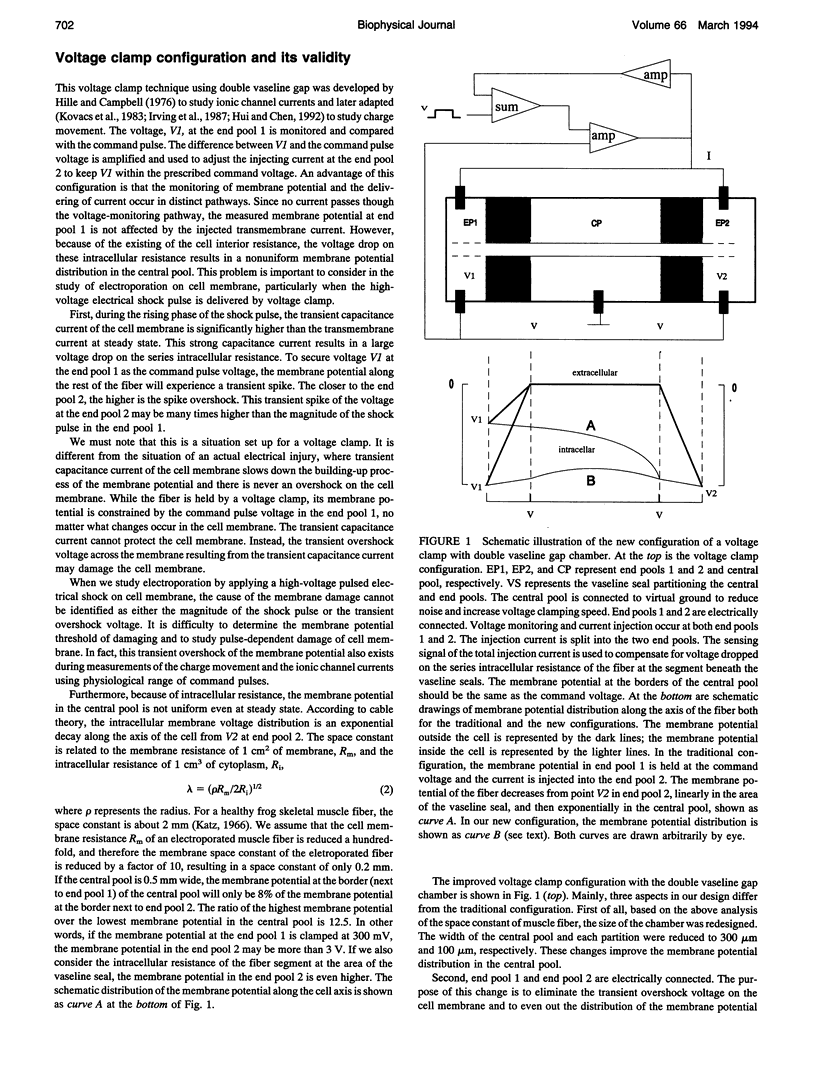
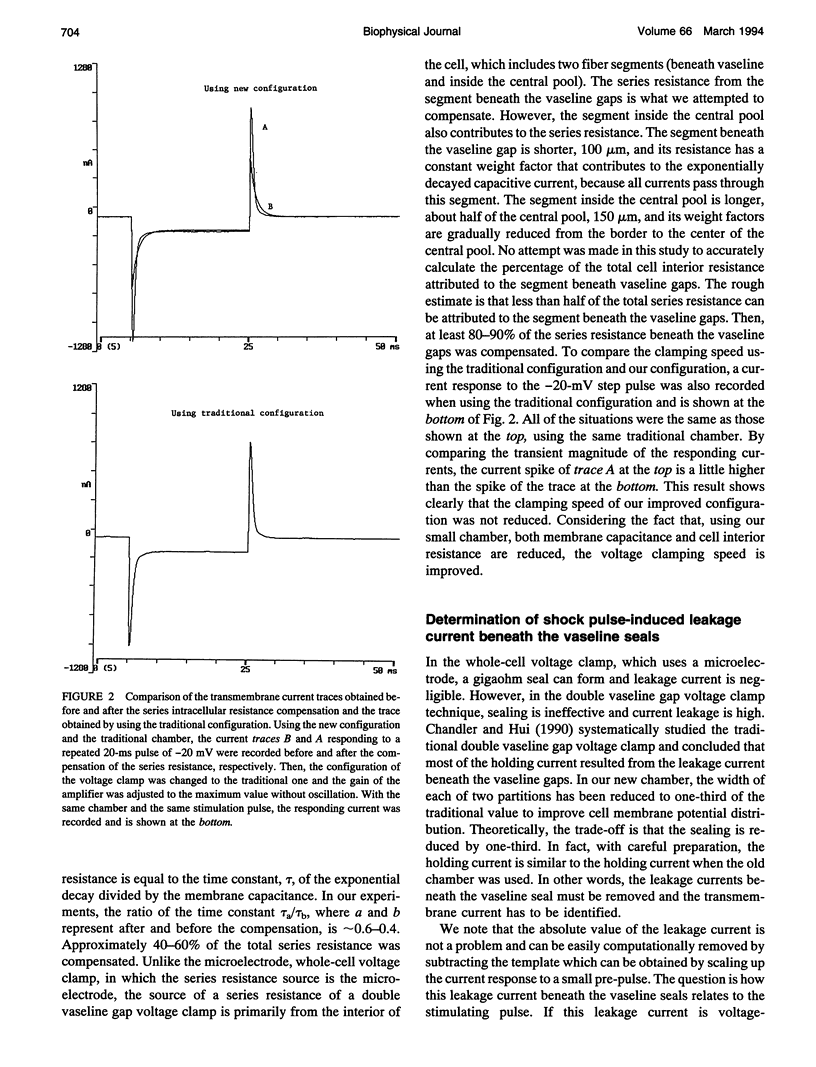
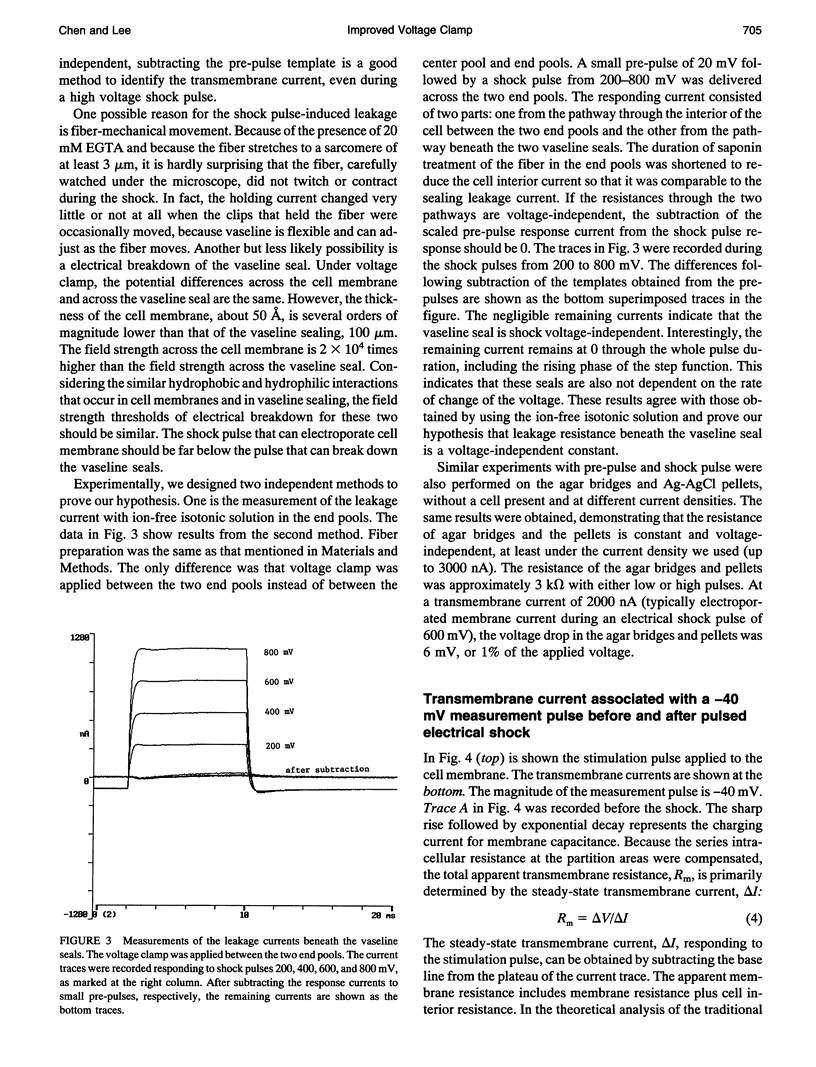
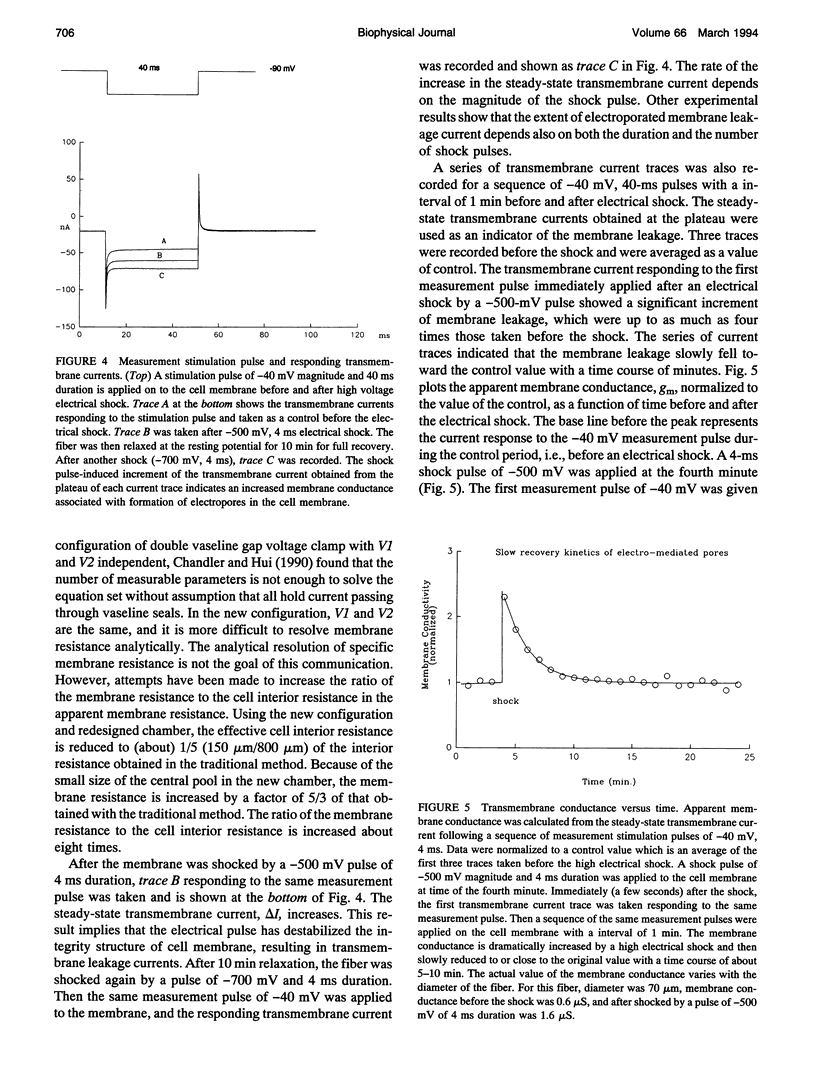
An improved voltage clamp with a double vaseline gap chamber was designed to study electroporated skeletal muscle fibers. The new clamp eliminated spike overshock of membrane potential when applying step stimulation occurring in the traditional configuration. It allowed greater consistency in membrane potential distribution. After the intracellular resistances of the fiber segment at the vaseline gap area were compensated, it was possible to change membrane potential more quickly. Using this technique, strong electrical pulses used to mimic the situation of electrical shock can be delivered to the cell membrane by voltage clamp. Transmembrane currents of skeletal muscle cell were simultaneously measured during a high pulsed shock and resolved into different components. Distinct transient changes of the transmembrane current, involving the time courses of the formation of electroporation and their recovery time constants, can be recorded. Because of more even membrane potential distribution and faster response to pulsed membrane potential change, this technique is also suitable for membrane study under physiological conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Zimmermann U. The resealing process of lipid bilayers after reversible electrical breakdown. Biochim Biophys Acta. 1981 Jan 8;640(1):169–178. doi: 10.1016/0005-2736(81)90542-3. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hui C. S. Membrane capacitance in frog cut twitch fibers mounted in a double vaseline-gap chamber. J Gen Physiol. 1990 Aug;96(2):225–256. doi: 10.1085/jgp.96.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. C., Gao P. Q., Maxwell B. L. High efficiency gene transfection by electroporation using a radio-frequency electric field. Biochim Biophys Acta. 1991 Apr 17;1092(2):153–160. doi: 10.1016/0167-4889(91)90149-r. [DOI] [PubMed] [Google Scholar]
- Chang D. C., Reese T. S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990 Jul;58(1):1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Hui C. S. Differential blockage of charge movement components in frog cut twitch fibres by nifedipine. J Physiol. 1991 Dec;444:579–603. doi: 10.1113/jphysiol.1991.sp018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L. V., Sukharev S. I., Popov S. V., Pastushenko V. F., Sokirko A. V., Abidor I. G., Chizmadzhev Y. A. The electrical breakdown of cell and lipid membranes: the similarity of phenomenologies. Biochim Biophys Acta. 1987 Sep 3;902(3):360–373. doi: 10.1016/0005-2736(87)90204-5. [DOI] [PubMed] [Google Scholar]
- Glaser R. W., Leikin S. L., Chernomordik L. V., Pastushenko V. F., Sokirko A. I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988 May 24;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Hibino M., Shigemori M., Itoh H., Nagayama K., Kinosita K., Jr Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys J. 1991 Jan;59(1):209–220. doi: 10.1016/S0006-3495(91)82212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui C. S., Chen W. Separation of Q beta and Q gamma charge components in frog cut twitch fibers with tetracaine. Critical comparison with other methods. J Gen Physiol. 1992 Jun;99(6):985–1016. doi: 10.1085/jgp.99.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. C., River L. P., Pan F. S., Ji L., Wollmann R. L. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4524–4528. doi: 10.1073/pnas.89.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. J., Tung L. Cell-attached patch clamp study of the electropermeabilization of amphibian cardiac cells. Biophys J. 1991 May;59(5):1028–1039. doi: 10.1016/S0006-3495(91)82318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Stimulation of a ouabain-sensitive Rb+ uptake in human erthrocytes with an external electric field. J Membr Biol. 1983;74(3):191–201. doi: 10.1007/BF02332123. [DOI] [PubMed] [Google Scholar]
- Teissie J., Tsong T. Y. Evidence of voltage-induced channel opening in Na/K ATPase of human erythrocyte membrane. J Membr Biol. 1980 Jul 15;55(2):133–140. doi: 10.1007/BF01871155. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegar R. A., Phillips J. W., Youngblom J. H., Morgan W. F. Cell electroporation is a highly efficient method for introducing restriction endonucleases into cells. Mutat Res. 1989 Jan-Feb;225(1-2):49–53. doi: 10.1016/0165-7992(89)90032-8. [DOI] [PubMed] [Google Scholar]
- Wong T. K., Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982 Jul 30;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]


