Abstract
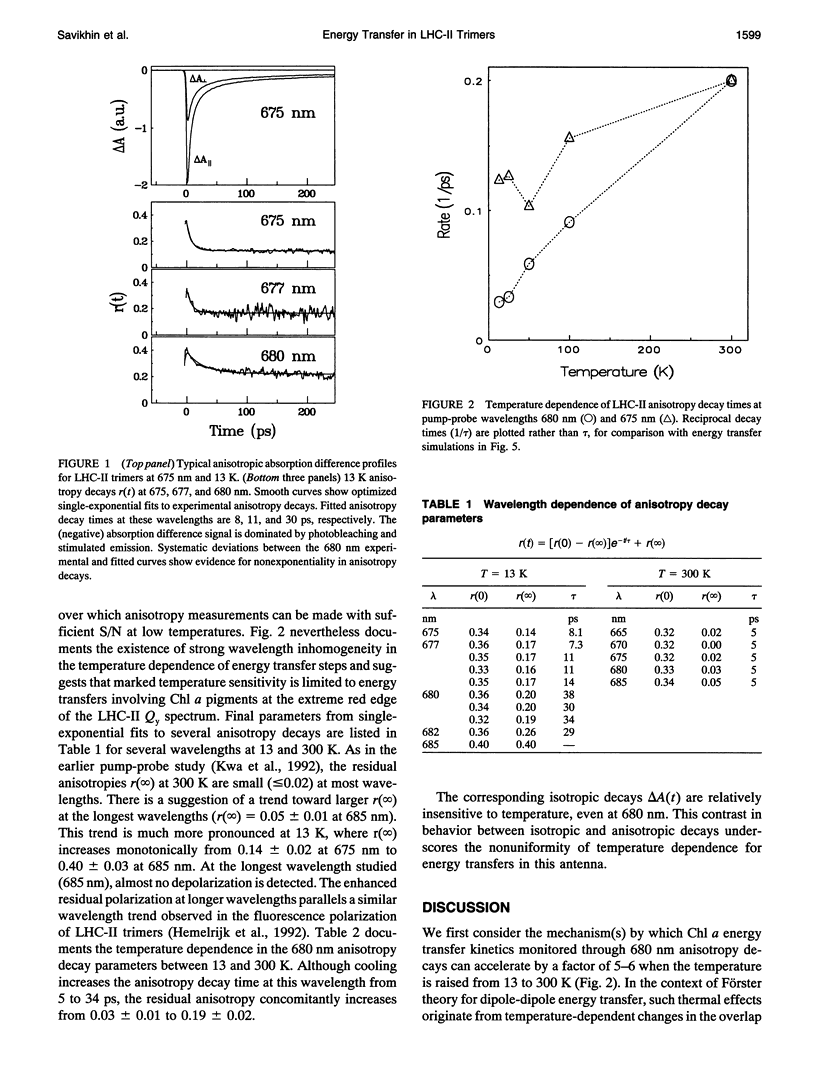
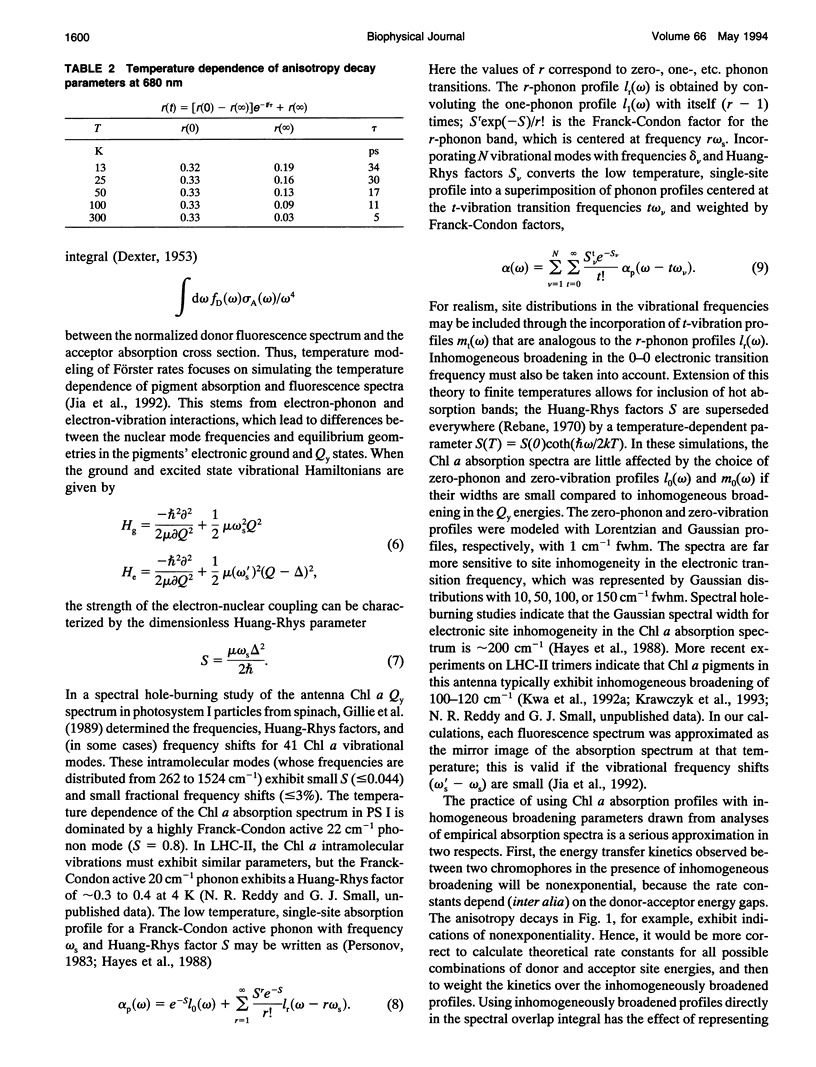
Temperature dependence in electronic energy transfer steps within light-harvesting antenna trimers from photosystem II was investigated by studying Chl a pump-probe anisotropy decays at several wavelengths from 675 to 682 nm. The anisotropy lifetime is markedly sensitive to temperature at the longest wavelengths (680-682 nm), increasing by factors of 5 to 6 as the trimers are cooled from room temperature to 13 K. The temperature dependence is muted at 677 and 675 nm. This behavior is modeled using simulations of temperature-broadened Chl a absorption and fluorescence spectra in spectral overlap calculations of Förster energy transfer rates. In this model, the 680 nm anisotropy decays are dominated by uphill energy transfers from 680 nm Chl a pigments at the red edge of the LHC-II spectrum; the 675 nm anisotropy decays reflect a statistical average of uphill and downhill energy transfers from 676-nm pigments. The measured temperature dependence is consistent with essentially uncorrelated inhomogeneous broadening of donor and acceptor Chl a pigments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. F. How does protein phosphorylation regulate photosynthesis? Trends Biochem Sci. 1992 Jan;17(1):12–17. doi: 10.1016/0968-0004(92)90418-9. [DOI] [PubMed] [Google Scholar]
- Jia Y., Jean J. M., Werst M. M., Chan C. K., Fleming G. R. Simulations of the temperature dependence of energy transfer in the PSI core antenna. Biophys J. 1992 Jul;63(1):259–273. doi: 10.1016/S0006-3495(92)81589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W., Downing K. H. Two-dimensional structure of plant light-harvesting complex at 3.7 A [corrected] resolution by electron crystallography. J Mol Biol. 1989 Jun 20;207(4):823–828. doi: 10.1016/0022-2836(89)90247-7. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W., Thaler T., Wehrli E. The structure of membrane crystals of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1983 May;96(5):1414–1424. doi: 10.1083/jcb.96.5.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N. Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature. 1991 Mar 14;350(6314):130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- Savikhin S., Wells T., Song P. S., Struve W. S. Ultrafast pump-probe spectroscopy of native etiolated oat phytochrome. Biochemistry. 1993 Jul 27;32(29):7512–7518. doi: 10.1021/bi00080a024. [DOI] [PubMed] [Google Scholar]


