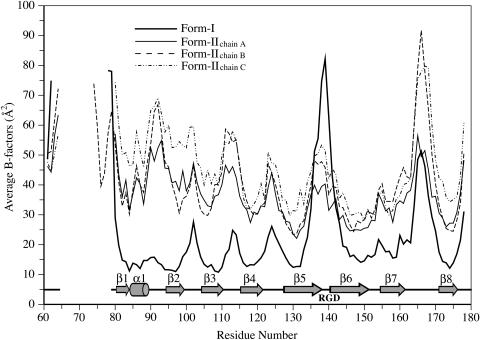
Figure 4.
Backbone Mobilities of ToxA.
The average main chain B-factors of form-I (thick line) and form-II chains A (thin line), B (dashed line), and C (dashed-dotted line) are plotted against residue numbers. Locations of the secondary structure elements and the RGD tripeptide are shown below the plot. As expected, loops and regions not stabilized by crystallographic packing interactions have B-factors higher than average, while β-strands forming the protein core have lower B-factors. For the loops with B-factors approaching 90 Å2, the most mobile residues have weak main chain density (<1 ρrms), but the chain path is still easily interpretable.