Figure 1.
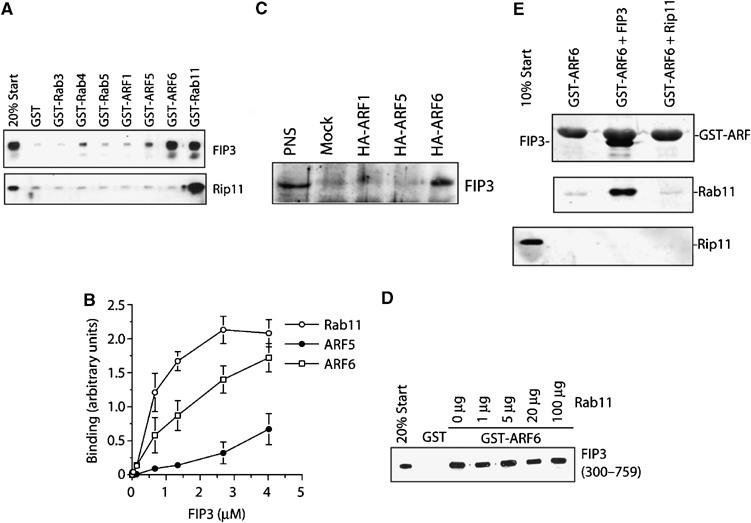
FIP3 binds Arf6 and Rab11. The binding characteristics of FIP3 were examined in a series of in vitro experiments. The C-terminus of FIP3 (residues 300–759) or Rip11 (residues 490–652) were expressed in bacteria as hexa–his fusions and incubated with 50 μl of beads loaded with 5 μg GST alone, or GST-Rabs/Arfs as indicated for 1 h. After washing, the beads were boiled in SDS–PAGE sample buffer and levels of associated FIP3 or Rip11 determined by immunoblotting. Data from a representative experiment is shown in (A). In the experiment shown in (B). increasing concentrations of FIP3-containing beads were incubated with 5 μg of Arf5, Arf6 or Rab11, and the binding determined by quantitative immunoblotting. The data shown is from three experiments of this type (mean±s.d.). (C) HeLa cells were transfected with HA-tagged Arf1, Arf5 or Arf6 as described. After 48 h, cells were lysed and an anti-HA immunoprecipitation performed. The immunoprecipitates were probed for the presence of endogenous FIP3. The result of a typical experiment is shown. In the experiment shown in (D), 50 μl of beads loaded with 5 μg of GST or GST-Arf6 were incubated with recombinant FIP3 in the presence of increasing amounts of Rab11. The ability of Rab11 to displace FIP3 from the Arf6-loaded beads was determined by immunoblotting. The experiment shown is typical of three experiments of this type. In order to determine whether FIP3 can form a ternary complex with Arf6 and Rab11, we performed the experiment shown in (E). GST-Arf6 was incubated with recombinant FIP3 (residues 300–759) or Rip11 (residues 490–652). The upper panel shows a Coomassie stain, revealing the interaction of FIP3 with Arf6, but not Rip11. These beads were incubated with recombinant Rab11, washed and then boiled in SDS–PAGE buffer and immunoblotted for Rab11 (middle panel) or Rip11 (lower panel).
