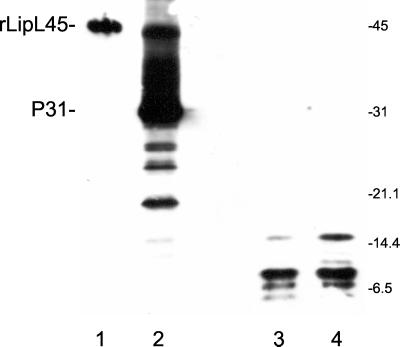
FIG. 6.
Peptide mapping of recombinant LipL45 and P31LipL45. Recombinant His6-LipL45 (rLipL45) and native P31LipL45 (P31) were gel purified, digested with V8 protease, and subjected to immunoblot analysis. The membrane was probed with serum generated against the C-terminal 88 amino acid residues of LipL45 (1:4,000). Lane 1, purified recombinant His6-LipL45; lane 2, high-passage L. kirschneri RM52; lane 3, gel-purified P31 treated with V8 protease; lane 4, gel-purified His6-LipL45 treated with V8 protease. The positions of molecular mass standards (in kilodaltons) are indicated on the right. The His6-LipL45 protein migrated as a 46-kDa band (lane 1). Note that LipL45 was detected when a large number of high-passage leptospires were loaded onto the gel (lane 2).