Figure 2.
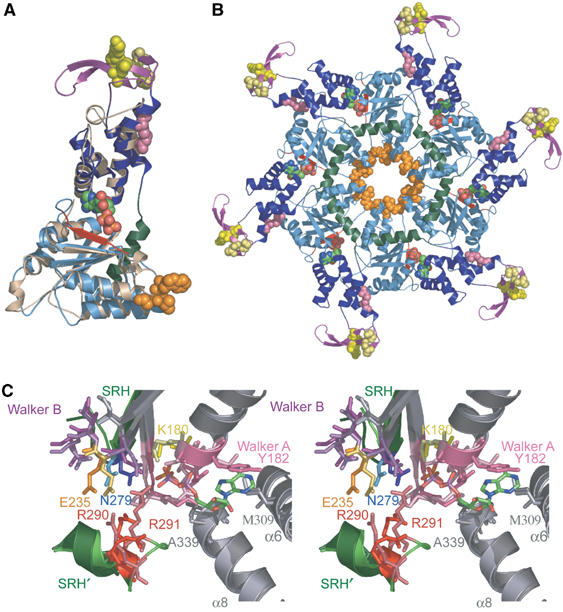
Structural similarities between hVPS4B123–444 and p97 D1. (A) Superposition of hVPS4B123–444 (color coded) onto ADP-bound p97 D1 protein (tan). Note that the individual domains of the AAA ATPase cassettes overlay very well, but that hVPS4B123–444 contains an additional N-terminal β-strand (red), an inserted β domain (purple), and an additional C-terminal helix (green). ADP (CPK atomic coloring) and the following residues are shown explicitly: pink; interface residue R354 (yR352); yellow, β domain residues K364 (yD362), L381 (yL373), S385 (yS377), D388 (yD380); orange, Pore 1 motif, 208WLG210 (y206WLG208). (B) Structural model for the hVPS4B123–444 ring. For clarity, the p97 hexamer used as an overlay template has been omitted, but the bound ADP molecule was retained. Residues in the two disordered loops of hVPS4B123–444 protein were modeled after those of the p97 D1 structure. Color coding matches panel A. (C) Stereoview of the overlaid nucleotide binding sites of p97 D1 (lighter shades) and hVPS4B (darker shades). Secondary structural elements that contribute to the active site are shown (gray), with the following highlights: Walker A motif (pink), Walker B motif (purple), the SRH β-strand (green), and the C-terminal SRH helix from an adjacent protomer (denoted SRH′, green). The positions of the bound ADP molecule from p97 and the sulfate ion from hVPS4B123–444 are shown. Key hVPS4B/p97 D1 residues shown explicitly are listed in the text, and the following residues (hVPS4B numbering) have been highlighted in unique colors: K180 (ATP binding, yellow), E235 (Mg2+ and ATP hydrolysis, gold), N279 (γ-phosphate sensing, blue), and R290–R291 (arginine fingers, red). Key residues in the two active sites are identical, except for a Ser to Thr substitution in the Walker A loop (181S in hVPS4B), and for conservative changes in the residues that stack on either side of the adenine ring (182Y and 309M in hVPS4B versus 253L and 380L in p97). A color-coded list of all active site residues is given in Supplementary Figure S1.
