Figure 6.
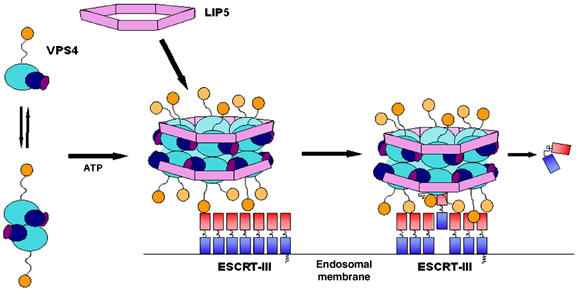
Model for the functional cycle of VPS4 proteins. Left: At steady state, hVPS4B is primarily a monomeric cytoplasmic protein (Fujita et al, 2004), and exhibits a monomer–dimer equilibrium in the absence of bound nucleotide (Babst et al, 1998; Supplementary Figure S3). LIP5/Vta1p is an oligomer of uncertain stoichiometry. Middle: Vps4 proteins are recruited to sites of vesicle formation at the endosomal membrane by interactions between the N-terminal MIT domain and the C-proximal domains of assembled ESCRT-III lattice/cage (Babst et al, 2002; Lin et al, 2005; Scott et al, 2005). The assembled Vps4 proteins can also bind ATP and LIP5/Vta1p oligomers via β domain interactions to form an enzymatically active complex. Note that a head-to-tail orientation of the two Vps4 rings (not shown) is equally consistent with our data. Right: We propose that bound ESCRT-III subunits are freed from the assembled lattice/cage and released into the cytoplasm as they are pulled up into the narrow central chamber of the hVPS4B ring.
