Figure 4.
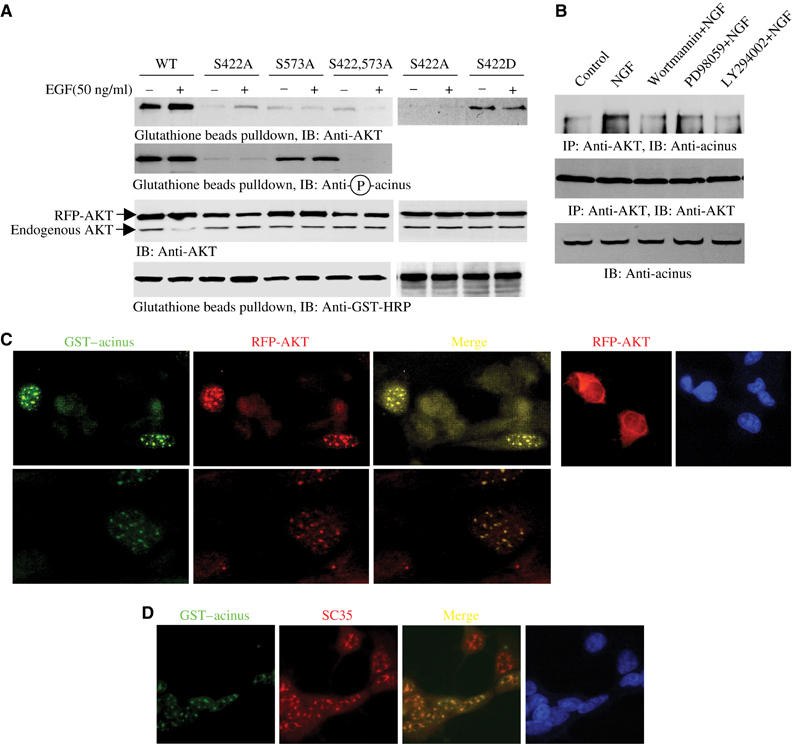
Akt binds to acinus. (A) Akt co-precipitates with GST–acinus-S. HEK 293 cells were cotransfected with RFP-Akt and GST–acinus-S wild type and mutants, followed by EGF stimulation. Akt strongly binds to acinus-S under basal condition. EGF enhanced the interaction. No significant interaction was observed between acinus mutants and Akt. Interestingly, S422D acinus binds to Akt, which was not regulated by EGF (top panel). S422 was potently phosphorylated in both wild-type and acinus (S573A) mutant (second panel). Equal amount of RFP-Akt and GST–acinus-S was employed (third and bottom panels). (B) Endogenous Akt binds to acinus-S. PC12 cells were respectively pretreated with various inhibitors for 30 min, then NGF was introduced. NGF elicited robust interaction between Akt and acinus-S, but PI3K inhibitors markedly blocked it. By contrast, MEK1 inhibitor had no effect (top panel). Equal amount of Akt was pulled down (middle panel). Equal level of acinus-S was employed (bottom panel). (C) Akt colocalizes with Acinus in the nucleus. HEK 293 cells were cotransfected with RFP-Akt and GST–acinus-S. The transfected cells were stained with anti-GST-FITC antibody. RFP-Akt colocalizes with GST–acinus in the nucleus (left three panels). RFP-Akt alone occurred in both the cytoplasm and the nucleus (right panels). (D) Akt and acinus-S colocalize in the nucleus with speckles. GST–acinus-transfected cells were stained with FITC-conjugated anti-GST antibody and anti-SC35, a specific marker for nuclear speckle.
