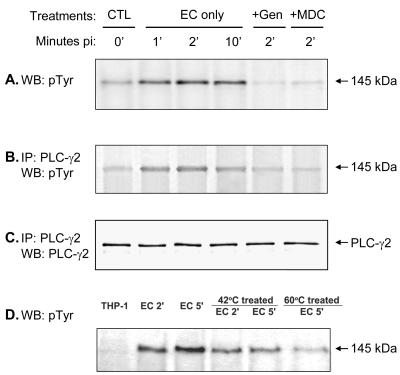
FIG. 5.
Tyrosine phosphorylation of PLC-γ2 in ehrlichial infection. (A) A 145-kDa protein was rapidly tyrosine phosphorylated in THP-1 cells upon binding of E. chaffeensis (EC). This protein was confirmed to be PLC-γ2 by immunoprecipitation (IP) with anti-PLC-γ2 antibody and by Western blot (WB) analysis with detection with anti-phosphotyrosine (pTyr) antibody (B) or anti-PLC-γ2 antibody (C). Tyrosine phosphorylation of PLC-γ2 was reduced when E. chaffeensis was pretreated at 42 or 60°C for 15 min (D). THP-1 cells were pretreated with genistein (Gen) or MDC for 1 h prior to the addition of E. chaffeensis. At different times, cells were lysed in RIPA buffer. Whole-cell lysates were immunoprecipitated with anti-PLC-γ2 antibody, and the immunoprecipitates or whole-cell lysates were subjected to Western blotting by using anti-phosphotyrosine antibody or anti-PLC-γ2 antibody. The results are representative of the results of more than four independent experiments in which similar results were obtained. CTL, control.