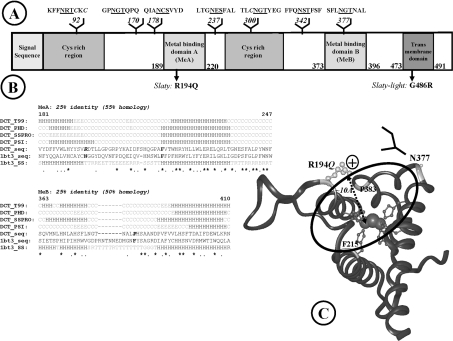
Figure 6. Modelling the active site containing the slaty mutation.
(A) Domain organization of proteins in the tyrosinase family. (B) Refined sequence alignment for metal-binding domains. Conserved histidine residues involved in metal binding are highlighted in grey; identical residues are marked with an asterisk, whereas similar residues are marked with a dot; the deleted loop from the template and the replacing potential N-glycosylation site (located on the edge of the binding site) are dot-underlined B, residue in isolated β bridge; E, extended β-strand; G, 310 helix; H, helix; S, bend; T, hydrogen-bonded turn; C, coil (other than helix and extended β in secondary structure prediction); R, random (not assigned secondary structure in the template). (C) Three-dimensional model of the active site of Dct. Histidine residues co-ordinating the metal ions are represented by ball-and-stick, coloured dark grey. Arg194 affected by the slaty mutation is light grey, the N-glycan replacing the loop in the template is indicated schematically by a branched structure. Arg194 is on the edge of the active-site cavity, at the appropriate distance for substrate binding.