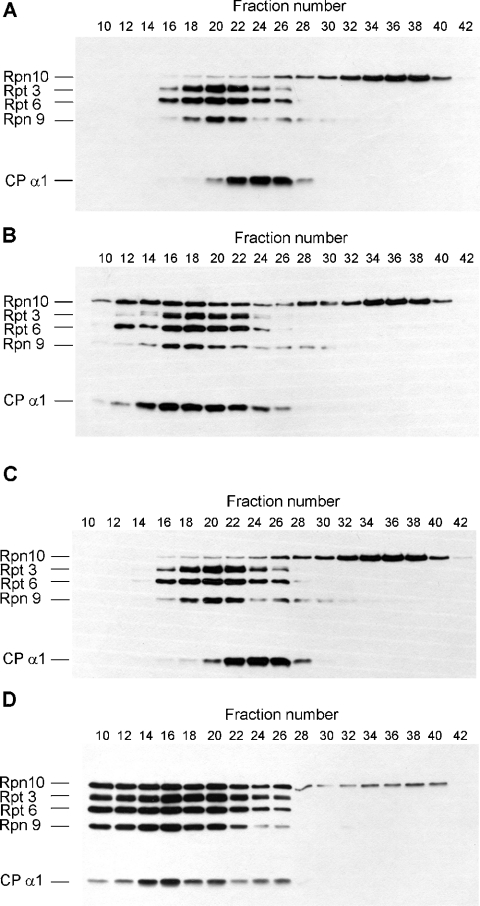
Figure 6. Hsp82 supports the reassembly of zinc-treated highly purified 26 S proteasome.
(A) Highly purified Drosophila 26 S proteasome was preincubated in the presence of ATP with 200 μM ZnCl2. After zinc removal, incubation was continued for an additional 20 min and the proteasomes were fractionated on Superose 6 sizing column. Column fractions (200 μl) were concentrated by precipitation with 7 vol. of acetone, run on 10% (w/v) SDS gel and immunoblotted with a mixture of five mAbs specific for subunits indicated on the left. (B) Highly purified Drosophila 26 S proteasome was preincubated in the presence of ATP with 200 μM ZnCl2. After the removal of zinc, incubation was continued with 5-fold molar excess of purified Hsp82 protein for an additional 20 min and the proteasomes were fractionated on Superose 6 sizing column. Column fractions (200 μl) were concentrated by precipitation with 7 vol. of acetone, run on 10% SDS gel and immunoblotted with a mixture of five mAbs specific for subunits indicated on the left. (C) DEAE-fractogel fraction of Drosophila 26 S proteasome was preincubated in the presence of ATP with 200 μM ZnCl2 and fractionated on Superose 6 sizing column in the presence of 200 μM ZnCl2. Aliquots (20 μl) of the column fraction were run on 10% SDS gel and immunoblotted with a mixture of five mAbs specific for subunits indicated on the left. (D) DEAE-fractogel fraction of Drosophila 26 S proteasome was preincubated in the presence of ATP with 200 μM ZnCl2. After zinc removal, incubation was continued for an additional 20 min and the proteasomes were fractionated on Superose 6 sizing column. Aliquots (20 μl) of the column fraction were run on 10% SDS gel and immunoblotted with a mixture of five mAbs specific for subunits indicated on the left.