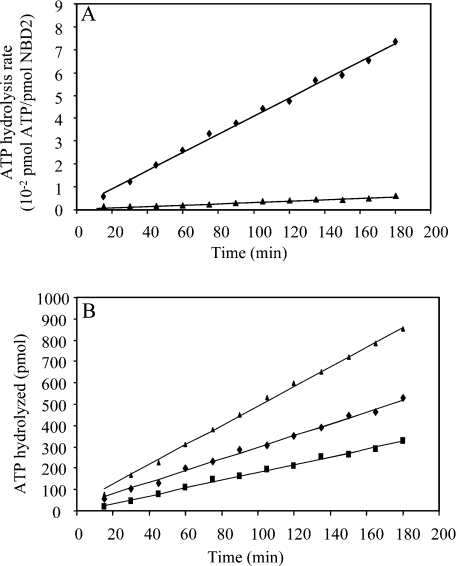
Figure 2. NBD2 and NBD1+NBD2 ATPase activity.
(A) NBD2 ATPase activity was determined using [γ-33P]ATP as substrate. The reaction mixtures contained NBD2 (66 μM) and ATP (1 mM) in 70 μl of buffer (50 mM Tris/HCl, pH 8 and 100 mM KCl) in the presence of 3 mM MgCl2 (◆) or 1 mM EDTA (▲). Aliquots (5 μl) were withdrawn at indicated times and [33P]Pi produced was counted. (B) ATPase activity of the NBDs was followed in standard buffer (50 mM Tris/HCl, pH 8, 100 mM KCl and 3 mM MgCl2) at 30 °C. The ATPase activity was measured as the amount of [γ-33P]ATP hydrolysed in aliquots (5 μl) at the indicated times and in the presence of 66 μM NBD2 (■); 66 μM NBD1 (◆); 66 μM NBD1 and 66 μM NBD2 (▲).