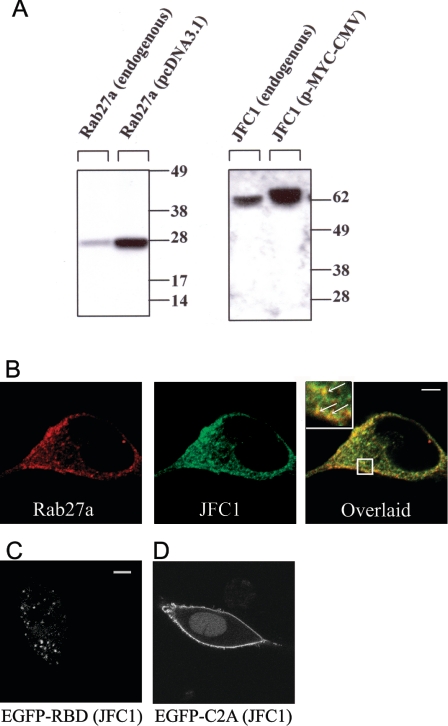
Figure 1. Expression and subcellular localization of JFC1 and Rab27a in LNCaP cells.
(A) LNCaP cells were transfected with the vectors pcDNA3.1-Rab27a, pCMV-myc-JFC1 or the corresponding empty vectors (endogenous). The cells were lysed and proteins (10 μg) were resolved by gel electrophoresis and transferred on to nitrocellulose membranes. JFC1 and Rab27a were detected by Western blotting using a rabbit polyclonal antibody raised against an N-terminal peptide of JFC1 and a mouse monoclonal antibody against Rab27a. The antibodies are described in the Experimental section. Molecular-mass sizes are given in kDa. (B) Immunofluorescence was performed in 3.7% (w/v) paraformaldehyde-fixed cells as described in the Experimental section. LNCaP cells were labelled for immunofluorescence detection of endogenous JFC1 (green) and endogenous Rab27a (red) using a mouse monoclonal antibody and a rabbit polyclonal antibody respectively. The cells were incubated with anti-JFC1 and anti-Rab27a antibodies or negative control (not shown) overnight at 4 °C. For detection, secondary antibodies Alexa-Fluor-conjugated donkey anti-mouse (488) and donkey anti-rabbit (594) were used. Cells were stored in Fluoromount-G (Southern Biotechnology) until analysed by laser-scanning confocal microscopy. (C and D) The chimaeras consisting of the EGFP–Rab-binding domain (RBD) of JFC1 (C) or the EGFP–C2A domain of JFC1 (D) were expressed in LNCaP cells. The cells were fixed 24–48 h after transfections and were analysed by confocal microscopy. Scale bars, 5 μm.