Abstract
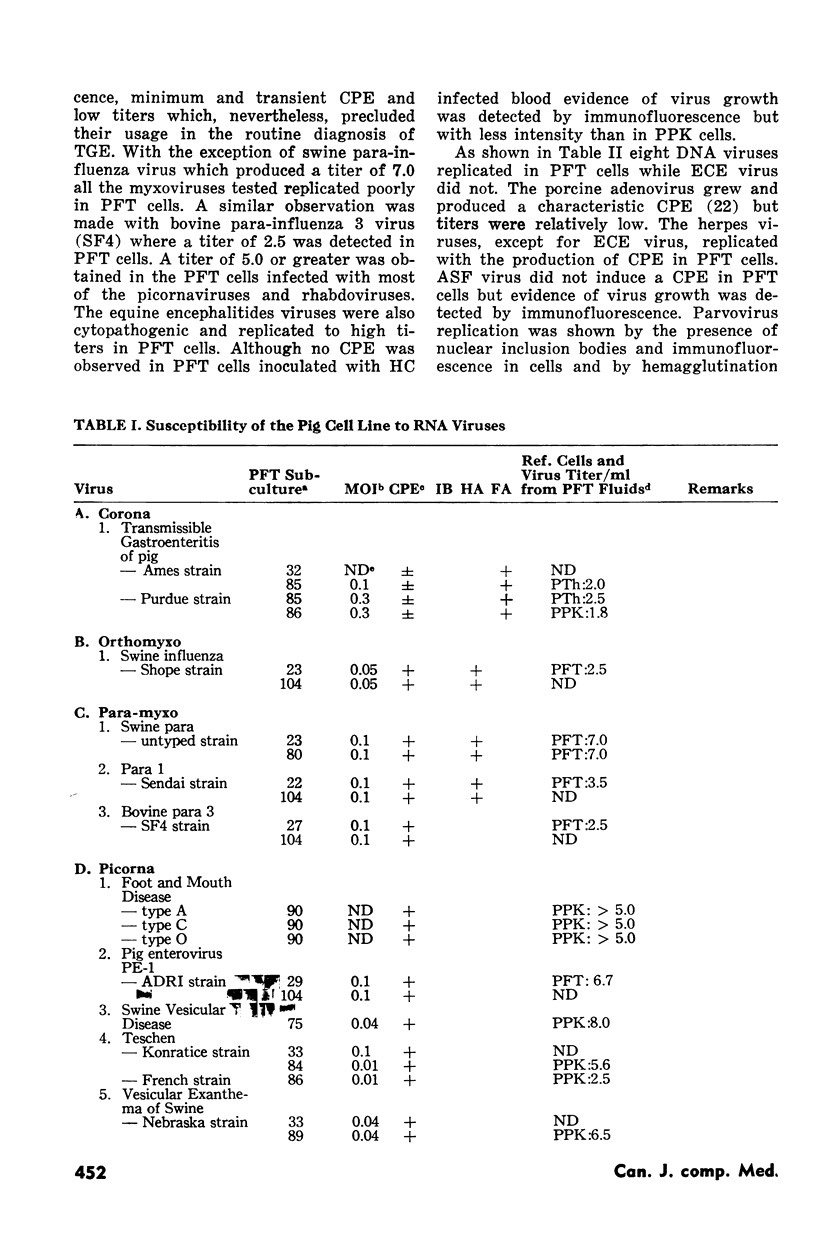
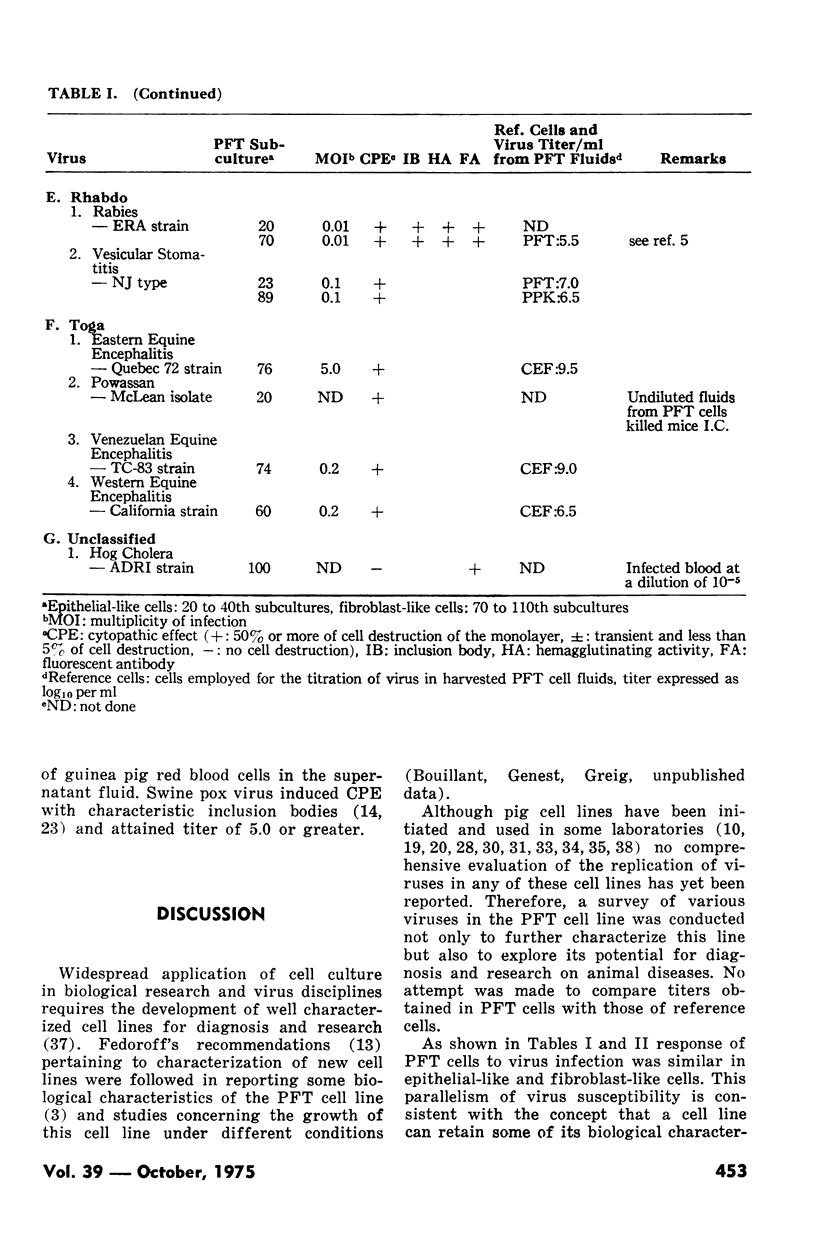
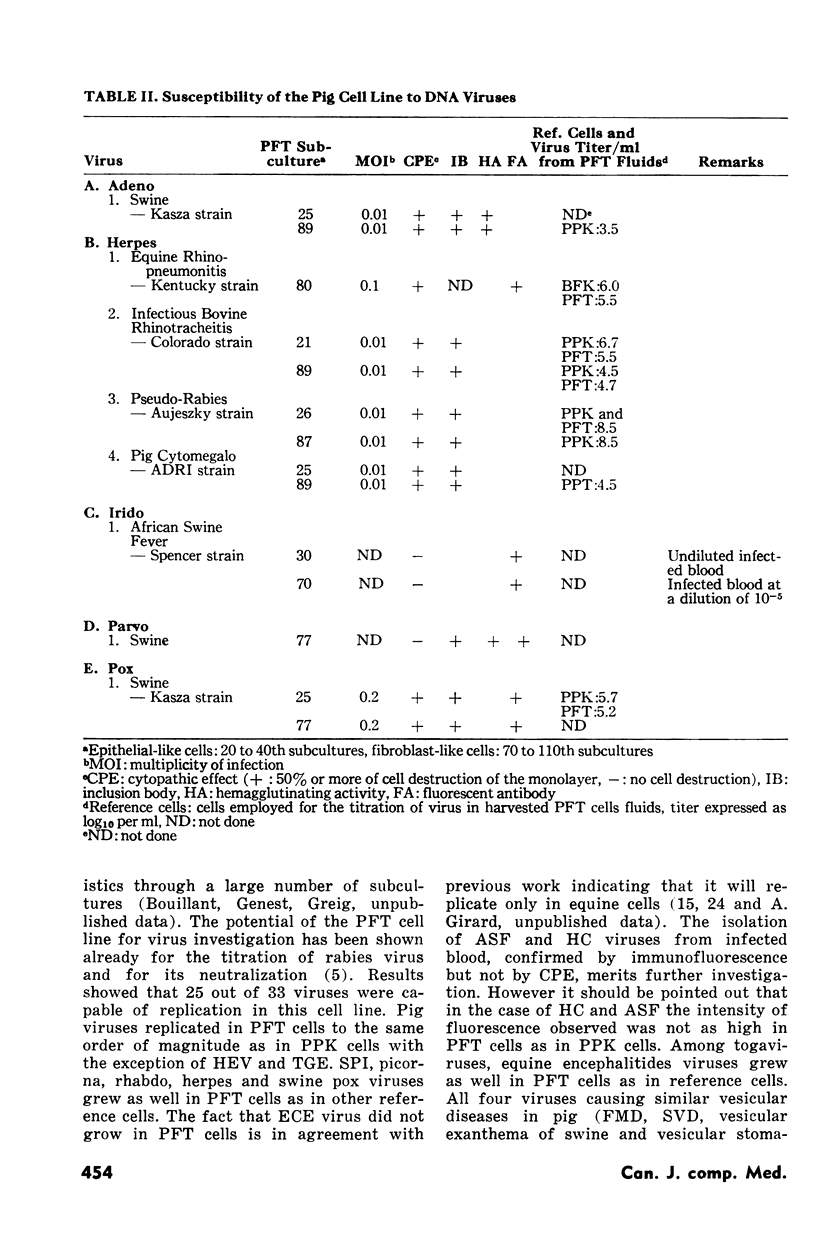
Seventeen of 24 RNA viruses and eight of nine DNA viruses replicated in a cell line derived from a pig fallopian tube. The following RNA viruses grew poorly in it: the virus of transmissible gastroenteritis of pig and the swine-influenza, Sendai and bovine para-influenza type 3 viruses. Among other RNA viruses an untyped swine para-myxovirus and some picornaviruses, rhabdoviruses and togaviruses attained high titers and produced an extensive cytopathic effect. Among the DNA viruses a porcine adeno, equine rhinopneumonitis, infectious bovine rhinotraceheitis, pseudorabies and porcine cytomegalo viruses replicated in pig fallopian tube cells as well as in other cells generally used to grow them.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Porterfield J. S., De Madrid A. T. C-type virus particles in pig kidney cell lines. J Gen Virol. 1971 Feb;10(2):195–198. doi: 10.1099/0022-1317-10-2-195. [DOI] [PubMed] [Google Scholar]
- Bouillant A. M., Greig A. S. Type C virus production by a continuous line of pig oviduct cells (PFT). J Gen Virol. 1975 May;27(2):173–180. doi: 10.1099/0022-1317-27-2-173. [DOI] [PubMed] [Google Scholar]
- Bouillant A. M., Tabel H., Greig A. S. Titration and neutralization of rabies virus (ERA strain) following its replication in a pig fallopian tube cell line. Can J Comp Med. 1974 Apr;38(2):118–123. [PMC free article] [PubMed] [Google Scholar]
- Bouliant A., Greig A. S., Genest P. Biological characterization of a cell line derived from the pig oviduct. In Vitro. 1973 Sep-Oct;9(2):92–102. doi: 10.1007/BF02616006. [DOI] [PubMed] [Google Scholar]
- Breese S. S., Jr Virus-like particles occurring in cultures of stable pig kidney cell lines. Brief report. Arch Gesamte Virusforsch. 1970;30(4):401–404. doi: 10.1007/BF01258369. [DOI] [PubMed] [Google Scholar]
- Croghan D. L., Matchett A., Koski T. A. Isolation of porcine parvovirus from commercial trypsin. Appl Microbiol. 1973 Sep;26(3):431–433. doi: 10.1128/am.26.3.431-433.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro M. P. An infectious agent causing "spontaneous" degeneration of swine cells in vitro. In Vitro. 1973 Jul-Aug;9(1):8–16. doi: 10.1007/BF02615982. [DOI] [PubMed] [Google Scholar]
- Dulac G. C., Boulanger P., Phaneuf J. B. Isolement du virus de la gastro-entérite transmissible du porc sur cultures cellulaires et comparaisons antigéniques avec deux souches américaines. Can Vet J. 1975 Mar;16(3):77–81. [PMC free article] [PubMed] [Google Scholar]
- GREIG A. S., BANNISTER G. L. INFECTION OF THE BOVINE UDDER WITH BOVINE HERPESVIRUS. Can J Comp Med Vet Sci. 1965 Mar;29:57–62. [PMC free article] [PubMed] [Google Scholar]
- Garg S. K., Meyer R. C. Adaptation of swinepox virus to an established cell line. Appl Microbiol. 1972 Jan;23(1):180–182. doi: 10.1128/am.23.1.180-182.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A., Greig A. S., Mitchell D. A virus associated with vulvitis and balanitis in the horse-- preliminary report. Can J Comp Med. 1968 Oct;32(4):603–604. [PMC free article] [PubMed] [Google Scholar]
- Greig A. S., Bannister G. L., Mitchell D., Corner A. H. Studies on Pathogenic Porcine Enteroviruses: II. Isolation of Virus in Tissue Culture from Brain and Feces of Clinical Cases. Can J Comp Med Vet Sci. 1961 Jun;25(6):142–150. [PMC free article] [PubMed] [Google Scholar]
- HAAG J., SANTUCCI J. EVOLUTION QUASI DIPLOUIDE D'UNE SOUCHE CELLULAIRE DE REIN DE PORC. Ann Genet. 1964;7:71–75. [PubMed] [Google Scholar]
- Hallauer C., Kronauer G., Siegl G. Parvoiruses as contaminants of permanent human cell lines. I. Virus isolation from 1960-1970. Arch Gesamte Virusforsch. 1971;35(1):80–90. doi: 10.1007/BF01249755. [DOI] [PubMed] [Google Scholar]
- Inoue Y. K., Yamada M. A. Clonal line of porcine kidney stable cells for assay of Japanese encephalitis virus. J Bacteriol. 1964 May;87(5):1239–1240. doi: 10.1128/jb.87.5.1239-1240.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASZA L., BOHL E. H., JONES D. O. Isolation and cultivation of swine pox virus in primary cell cultures of swine origin. Am J Vet Res. 1960 Mar;21:269–273. [PubMed] [Google Scholar]
- Kasza L. Isolation of an adenovirus from the brain of a pig. Am J Vet Res. 1966 May;27(118):751–758. [PubMed] [Google Scholar]
- Krogsrud J., Onstad O. Equine coital exanthema. Isolation of a virus and transmission experiments. Acta Vet Scand. 1971;12(1):1–14. [PubMed] [Google Scholar]
- Lieber M. M., Benveniste R. E., Livingston D. M., Todaro G. J. Mammalian cells in culture frequently release type C viruses. Science. 1973 Oct 5;182(4107):56–59. doi: 10.1126/science.182.4107.56. [DOI] [PubMed] [Google Scholar]
- Molander C. W., Kniazeff A. J., Boone C. W., Paley A., Imagawa D. T. Isolation and characterization of viruses from fetal calf serum. In Vitro. 1971 Nov-Dec;7(3):168–173. doi: 10.1007/BF02617962. [DOI] [PubMed] [Google Scholar]
- Morin M., Morehouse L. G., Solorzano R. F., Olson L. D. Transmissible gastroenteritis in feeder swine: clinical, immunofluorescence and histopathological obervations. Can J Comp Med. 1973 Jul;37(3):239–248. [PMC free article] [PubMed] [Google Scholar]
- Petricciani J. C., Hopps H. E., Lorenz D. E. Subhuman primate diploid cells: possible substrates for production of virus vaccines. Science. 1971 Dec 3;174(4013):1025–1027. doi: 10.1126/science.174.4013.1025. [DOI] [PubMed] [Google Scholar]
- Pirtle E. C., Mengeling W. L., Cheville N. F. Initiation and characterization of two porcine embryonal nephroma cell lines. Am J Vet Res. 1970 Sep;31(9):1601–1608. [PubMed] [Google Scholar]
- RUDDLE F. H. Chromosome variation in cell populations derived from pig kidney. Cancer Res. 1961 Aug;21:885–894. [PubMed] [Google Scholar]
- Shimizu Y., Furuuchi S., Hayashi S., Kumagai T., Sasahara J. Porcine kidney cell line persistently contaminated with avirulent swine fever virus. J Gen Virol. 1969 Jun;4(4):625–628. doi: 10.1099/0022-1317-4-4-625. [DOI] [PubMed] [Google Scholar]
- Stulberg C. S., Coriell L. L., Kniazeff A. J., Shannon J. E. The animal cell culture collection. In Vitro. 1970;5:1–16. doi: 10.1007/BF02618370. [DOI] [PubMed] [Google Scholar]
- Westaway E. G. Assessment and application of a cell line from pig kidney for plaque assay and neutralization tests with twelve group B arboviruses. Am J Epidemiol. 1966 Nov;84(3):439–456. doi: 10.1093/oxfordjournals.aje.a120657. [DOI] [PubMed] [Google Scholar]


