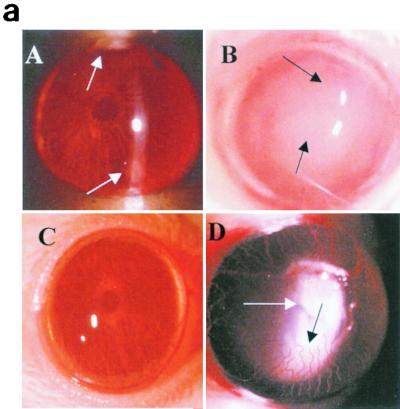
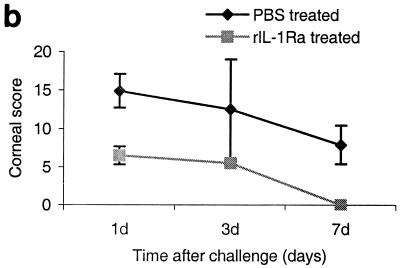
FIG. 5.
(a) Clinical examination of corneas injected with 20 μl of rIL-1Ra (20 μg during each injection) subconjunctivally at 24 h and then 3 h before infection with the invasive strain. Control mice received an equal volume of PBS at same time points before the infection with invasive strain. Panel A, rIL-1Ra-treated mice at 24 h postchallenge, showing focal infiltrates at the periphery of the cornea (arrows). Panel B, control mice showed extensive infiltration of inflammatory cells (arrow) in the central cornea at 24 h postchallenge, with severe edema and moderate anterior chamber response. Panel C, rIL-1Ra-treated mice at 7 days postchallenge; infiltrates have completely resolved. Panel D, in control mice the epithelium had healed but a large number of infiltrates were still present (white arrow), and new vessel growth was evident (black arrow). (b) Composite clinical scores (composed of the graded density, depth, and extent of corneal infiltrates as well as size and depth of corneal ulceration; see Table 1 for the grading system) at 1, 3, and 7 days postchallenge for corneas treated with 20 μl of rIL-1Ra (20 μg during each injection) subconjunctivally at 24 and 3 h before the infection with invasive strain. Control mice received an equal volume of PBS at same time points before the infection with invasive strain. Error bars indicate standard errors of the means.