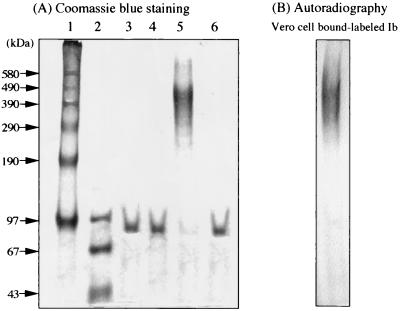
FIG. 1.
Purification of rIb from GST-Ib. (A) Chymotrypsin-treated GST-Ib purified by glutathione-Sepharose column chromatography was applied to a Mono-Q column and was eluted with a 0 to 1.0 M NaCl gradient in 20 mM Tris-HCl buffer (pH 8). The first and second fractions were analyzed by SDS-3.5% PAGE with incubation at 37°C for 15 min or with heating at 95°C for 3 min. Protein (10 μg each) was stained with Coomassie blue. Lane 1, high-molecular-mass standard (phosphorylase b cross-linked; Sigma); lane 2, low-molecular-mass standard (Pharmacia); lane 3, first fraction (37°C, 15 min); lane 4, first fraction (95°C, 3 min); lane 5, second fraction (37°C, 15 min); and lane 6, second fraction (95°C, 3 min). (B) Vero cells were incubated with 125I-labeled Ib at 37°C for 60 min, washed, dissolved in SDS-sample buffer, and subjected to SDS-3.5% PAGE prior to incubation at 37°C for 15 min. After electrophoresis, the gel was dried and autoradiographed. The radiolabeled band was analyzed with a Fuji BAS 2000 system.