Abstract
1. In cats, extracellular micro-electrode recordings were made from axons of the spinocervical tract (s.c.t.) in both the decerebrate state and during cold block of the spinal cord (reversible spinal state) to examine the effects of intra-arterial injection of algesic agents (bradykinin, potassium, 5-hydroxytryptamine) into the gastrocnemius-soleus (g.s.) muscle on the discharge behaviour of s.c.t. neurones.
2. In the decerebrate state without cooling the spinal cord 13% of the cells (eleven out of eighty-three) responded to intra-arterial injection of bradykinin, 33% (twenty-two out of sixty-nine) to 5-hydroxytryptamine, and 38% (thirty-five out of ninety-one) to potassium injection.
3. The general time course and the latency of the responses of s.c.t. cells induced by injection of pain-producing substances into the g.s. muscle reflect in many respects the activations of g.s. group III and group IV primary afferent units studied previously.
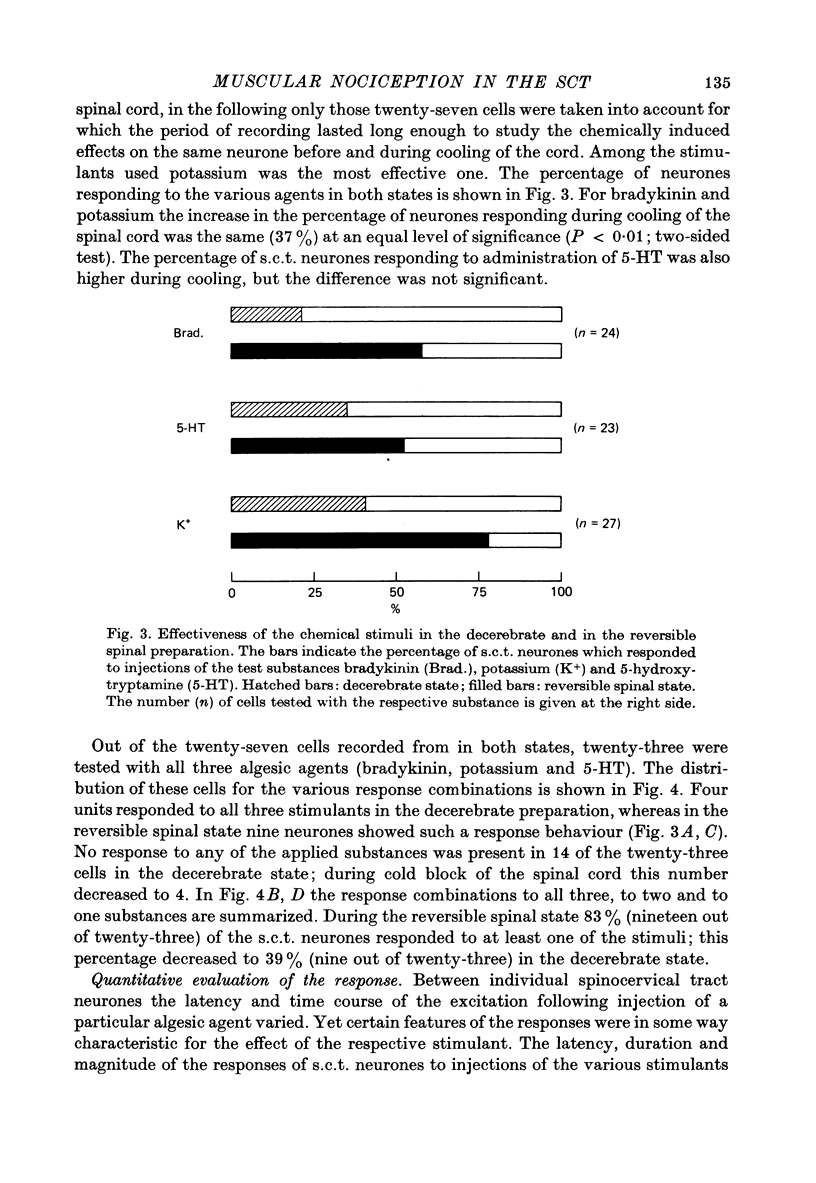
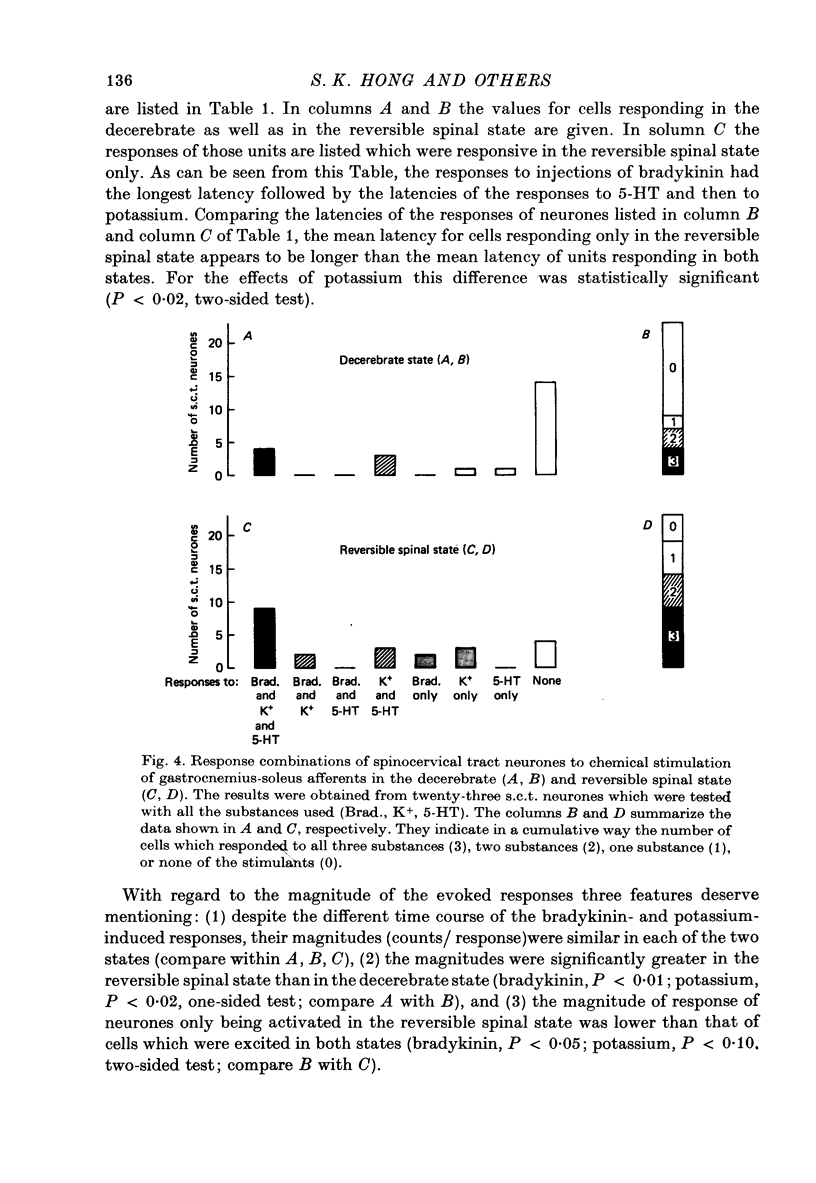
4. For twenty-seven s.c.t. neurones the period of recording was long enough to record the responses of the same cell to injections of algesic agents in both the decerebrate and the reversible spinal state. In the reversible spinal state 83% (nineteen out of twenty-three) of the s.c.t. neurones tested with all the three substances responded to at least one of the algesic agents. In the decerebrate state the percentage was lower (39%).
5. Reversible spinalization led not only to a significant increase in the number of s.c.t. neurones responding to the algesic agents used but also to an increase in the magnitude of the chemically induced responses.
6. The mean latency of the responses of neurones that were activated in both preparations were shorter in the reversible spinal state than in the decerebrate state.
7. Control experiments showed that the responses to bradykinin and potassium were entirely due to the nervous outflow from the g.s. muscle. In contrast, intra-arterially applied 5-hydroxytryptamine influenced the s.c.t. cells via unknown additional sites of action.
8. The results indicate that muscular group III and/or group IV units excitable by algesic substances do project on to neurones of the spinocervical tract. Furthermore it is concluded that the responses of s.c.t. neurones to activation of fine muscle afferents by algesic agents are subject to a descending control similar to the well known descending modulation of their responsiveness to cutaneous input. Therefore, in addition to serving as a cutaneous pathway the spinocervical tract may take part in muscular nociception.
Full text
PDF



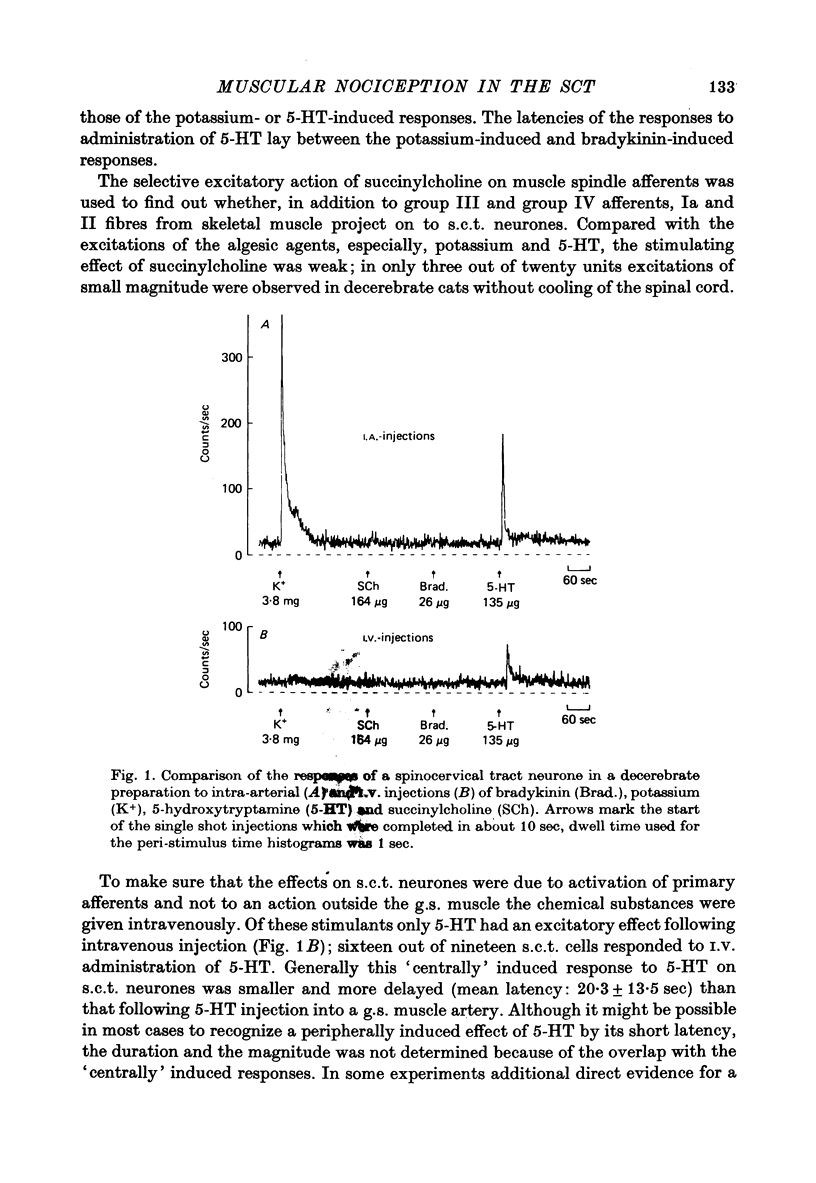
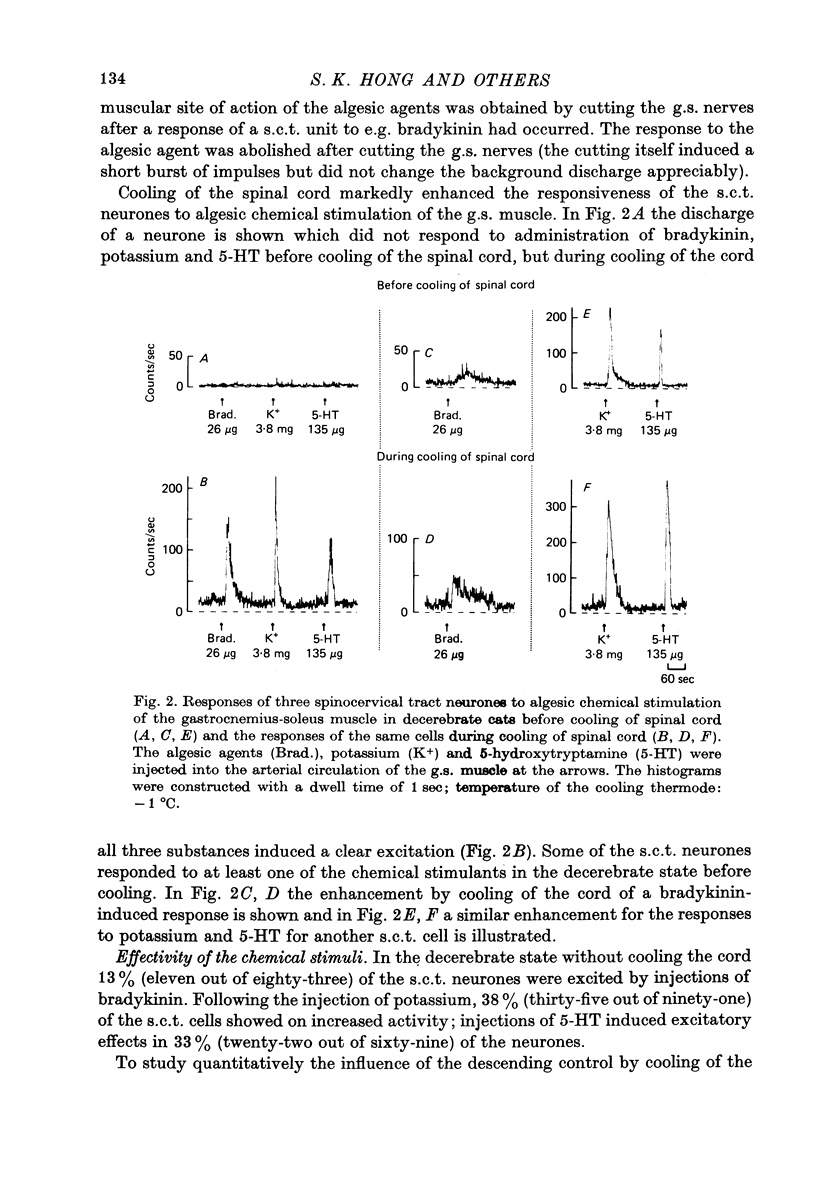




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BESSOU P., LAPORTE Y. Activation des fibres afférentes amyéliniques d'origine musculaire. C R Seances Soc Biol Fil. 1958;152(11):1587–1590. [PubMed] [Google Scholar]
- Besson J. M., Conseiller C., Hamann K. F., Maillard M. C. Modifications of dorsal horn cell activities in the spinal cord, after intra-arterial injection of bradykinin. J Physiol. 1972 Feb;221(1):189–205. doi: 10.1113/jphysiol.1972.sp009748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson J. M., Guilbaud G., Le Bars D. Descending inhibitory influences exerted by the brain stem upon the activities of dorsal horn lamina V cells induced by intra-arterial injection of bradykinin into the limbs. J Physiol. 1975 Jul;248(3):725–739. doi: 10.1113/jphysiol.1975.sp010997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G. Descending control of the spinocervical tract in decerebrate cats. Brain Res. 1970 Jan 6;17(1):152–155. doi: 10.1016/0006-8993(70)90319-7. [DOI] [PubMed] [Google Scholar]
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Hamann W. C., Martin H. F., 3rd Effects of activity in non-myelinated afferent fibres on the spinocervical tract. Brain Res. 1975 Nov 14;98(2):243–259. doi: 10.1016/0006-8993(75)90004-9. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Kirk E. J., Martin H. F., 3rd Descending and segmental inhibition of transmission through the spinocervical tract. J Physiol. 1973 May;230(3):689–705. doi: 10.1113/jphysiol.1973.sp010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Martin H. F., 3rd Activation of descending control of the spinocervical tract by impulses ascending the dorsal columns and relaying through the dorsal column nuclei. J Physiol. 1973 Dec;235(2):535–550. doi: 10.1113/jphysiol.1973.sp010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol. 1977 May;267(2):537–558. doi: 10.1113/jphysiol.1977.sp011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E. Pyramidal tract effects on interneurons in the cat lumbar dorsal horn. J Neurophysiol. 1968 Jan;31(1):69–80. doi: 10.1152/jn.1968.31.1.69. [DOI] [PubMed] [Google Scholar]
- Fock S., Mense S. Excitatory effects of 5-hydroxytryptamine, histamine and potassium ions on muscular group IV afferent units: a comparison with bradykinin. Brain Res. 1976 Apr 9;105(3):459–469. doi: 10.1016/0006-8993(76)90593-x. [DOI] [PubMed] [Google Scholar]
- Foreman R. D., Kenshalo D. R., Jr, Schmidt R. F., Willis W. D. Field potentials and excitation of primate spinothalamic neurones in response to volleys in muscle afferents. J Physiol. 1979 Jan;286:197–213. doi: 10.1113/jphysiol.1979.sp012614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman R. D., Schmidt R. F., Willis W. D. Effects of mechanical and chemical stimulation of fine muscle afferents upon primate spinothalamic tract cells. J Physiol. 1979 Jan;286:215–231. doi: 10.1113/jphysiol.1979.sp012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M., Mense S. Muscle receptors with group IV afferent fibres responding to application of bradykinin. Brain Res. 1975 Jul 18;92(3):369–383. doi: 10.1016/0006-8993(75)90323-6. [DOI] [PubMed] [Google Scholar]
- GRANIT R., SKOGLUND S., THESLEFF S. Activation of muscle spindles by succinylcholine and decamethonium, the effects of curare. Acta Physiol Scand. 1953;28(2-3):134–151. doi: 10.1111/j.1748-1716.1953.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Hamann W. C., Hong S. K., Kniffki K. D., Schmidt R. F. Projections of primary afferent fibres from muscle to neurones of the spinocervical tract of the cat. J Physiol. 1978 Oct;283:369–378. doi: 10.1113/jphysiol.1978.sp012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker H. O., Iggo A., Zimmermann M. Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimuli. Pain. 1975 Jun;1(2):147–165. doi: 10.1016/0304-3959(75)90099-8. [DOI] [PubMed] [Google Scholar]
- Hillman P., Wall P. D. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res. 1969;9(4):284–306. doi: 10.1007/BF00235240. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977 Dec;273(1):179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A., NORRSELL U., VOORHOEVE P. EFFECTS FROM THE SENSORIMOTOR CORTEX ON ASCENDING SPINAL PATHWAYS. Acta Physiol Scand. 1963 Dec;59:462–473. doi: 10.1111/j.1748-1716.1963.tb02762.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Three ascending spinal pathways in the dorsal part of the lateral funiculus. Acta Physiol Scand. 1961 Jan;51:1–16. doi: 10.1111/j.1748-1716.1961.tb02108.x. [DOI] [PubMed] [Google Scholar]
- LeBars D., Menetrey D., Besson J. M. Effects of morphine upon the lamina V type cells activities in the dorsal horn of the decerebrate cat. Brain Res. 1976 Aug 27;113(2):293–310. doi: 10.1016/0006-8993(76)90942-2. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966 Nov;16(3):316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrsell U., Wolpow E. R. An evoked potential study of different pathways from the hindlimb to the somatosensory areas in the cat. Acta Physiol Scand. 1966 Jan-Feb;66(1):19–33. doi: 10.1111/j.1748-1716.1966.tb03164.x. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz B., Wall P. D., Weber W. V. Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol. 1968 Dec;199(3):511–532. doi: 10.1113/jphysiol.1968.sp008666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUB A., BISHOP P. O. THE SPINOCERVICAL TRACT: DORSAL COLUMN LINKAGE, CONDUCTION VELOCITY, PRIMARY AFFERENT SPECTRUM. Exp Neurol. 1965 Sep;13:1–21. doi: 10.1016/0014-4886(65)90002-6. [DOI] [PubMed] [Google Scholar]
- TAUB A. LOCAL, SEGMENTAL AND SUPRASPINAL INTERACTION WITH A DORSOLATERAL SPINAL CUTANEOUS AFFERENT SYSTEM. Exp Neurol. 1964 Oct;10:357–374. doi: 10.1016/0014-4886(64)90006-8. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren B. G. Habituation of spinal interneurons. J Neurophysiol. 1967 Nov;30(6):1424–1438. doi: 10.1152/jn.1967.30.6.1424. [DOI] [PubMed] [Google Scholar]


